You are here: Home > Office of the Clinical Director
Office of the Clinical Director

- Stephen G. Kaler, MD, MPH, Clinical Director
- Linda Aspinall, Protocol Coordinator
- DuShon Hutchinson, Patient Care Coordinator
- Nghi Huynh, Patient Specimen Coordinator
- Maryellen Rechen, BS, RN, Special Assistant to the Clinical Director
Clinical Investigation Across the Lifespan
The NICHD intramural clinical research program currently includes 114 protocols with five main areas of focus: 1) adult, pediatric, and reproductive endocrinology, 2) human genetics, 3) normal growth and development, 4) national/international public health, and 5) women’s health (Figures 1–3). The conduct of studies that this portfolio comprises is guided by two entities administered by the Office of the Clinical Director: the NICHD Institutional Review Board (IRB) and the NICHD Data Safety Monitoring Committee (DSMC), which possess expertise in issues unique to clinical investigation in infants, children, adolescents, and adult women and men. The composition of the IRB is diverse in terms of medical expertise and NIH Institutes and Centers (less than 30% percent of members are affiliated with NICHD). Clinical investigators express very high satisfaction with the NICHD IRB, and our program is thriving, with 24% growth in the number of protocols in the last five years.
Members of the NICHD DSMC include Dr. David DeMets and Dr. Lawrence Friedman, co-authors of the textbook Fundamentals of Clinical Trials. The DSMC has the general charge of ensuring, through advice to the investigators and the NICHD Clinical Director, that clinical trials are conducted safely and ethically and that the trial meets its primary objectives. The DSMC must consider the interests of currently enrolled patients as well as the interests of patients to be enrolled in the future and patients outside the study. On a schedule determined before data are collected, the DSMC examines prospectively selected outcome data for early evidence of efficacy or lack thereof. Throughout the trial, the committee monitors study assumptions about incidence rates and sample size. The DSMC evaluates outcome data according to established guidelines for data monitoring. Based on data reviewed at these interim evaluations, the committee may recommend to the NICHD and its IRB early termination of a trial either because of established efficacy of treatment or because of the unlikelihood that a meaningful assessment of treatment effect could be established by the planned end of the trial. Stopping rules are specified in advance of the analysis and agreed upon by the DSMC, the investigator, and the NICHD IRB. The DSMC may also recommend to the PI and IRB extensions in trial length or increases in sample size, as well as other relevant modifications to the protocol.
Over 135 publications emanated from NICHD clinical protocols during the past year, including six articles in the New England Journal of Medicine.
Contact
For more information, email sgk@box-s.nih.gov or visit icrp.nichd.nih.gov.
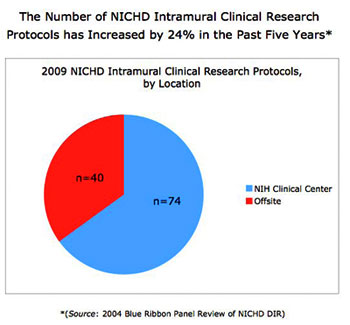 Figure 1. Growth of the NICHD Intramural Clinical Research Program
Figure 1. Growth of the NICHD Intramural Clinical Research Program
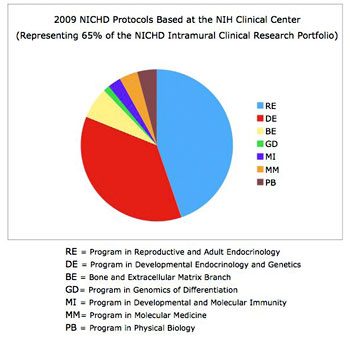 Figure 2. NICHD Clinical Research at the NIH Clinical Center
Figure 2. NICHD Clinical Research at the NIH Clinical Center
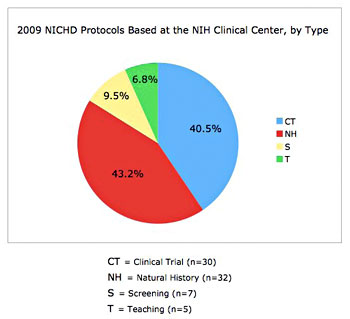 Figure 3. The NICHD Clinical Research Portfolio at the Clinical Center
Figure 3. The NICHD Clinical Research Portfolio at the Clinical Center



