You are here: Home > Office of the Clinical Director
Office of the Clinical Director

- Forbes D. Porter, MD, PhD, Clinical Director
- Linda Aspinall, Protocol Coordinator
- DuShon Hutchinson, Patient Care Coordinator
- Nghi Huynh, Patient Specimen Coordinator
- Maryellen Rechen, BS, RN, Special Assistant to the Clinical Director
Clinical Investigation Across the Lifespan
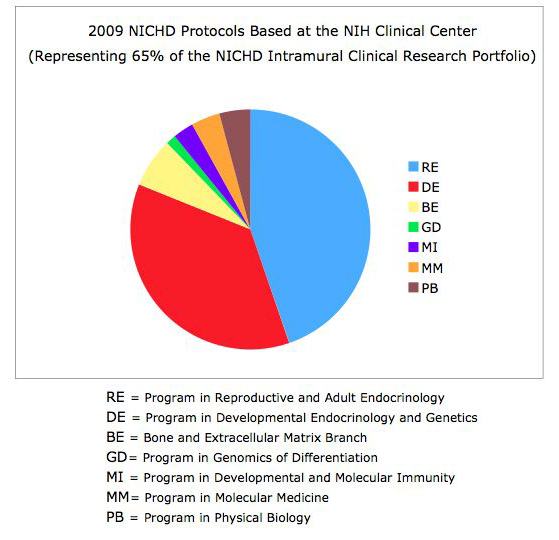
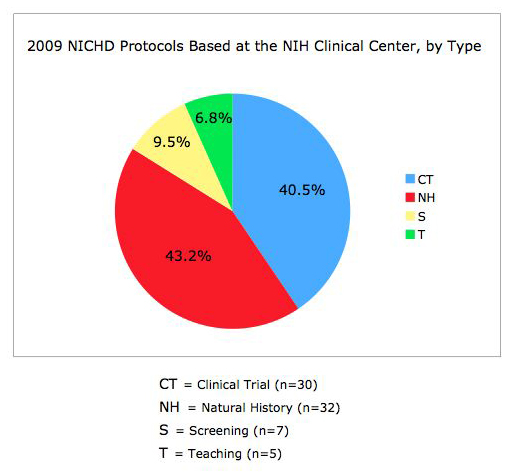
The NICHD intramural clinical research program currently includes 115 protocols with five main areas of focus: 1) adult, pediatric, and reproductive endocrinology, 2) human genetics, 3) normal growth and development, 4) national/international public health, and 5) women's health (Figures 1 and 2). The conduct of studies that this portfolio comprises is guided by two entities administered by the Office of the Clinical Director: the NICHD Institutional Review Board (IRB) and the NICHD Data Safety Monitoring Committee (DSMC). The NICHD Office of the Clinical Director supports clinical protocols that involve 28 Investigational New Drugs/Devices, including development of new vaccines. The NICHD clinical research program has grown by 25% over the past five years.
The NICHD IRB is chaired by Gilman Grave and has 16 members, of which three joined in the past year. The composition of the NICHD IRB is diverse, both in terms of medical and ethical expertise and affiliation. The NICHD DSMC is chaired by Dr. Frank Pucino, and membership includes Drs. David DeMets and Lawrence Friedman, co-authors of the textbook Fundamentals of Clinical Trials. Both committees possess expertise in issues related to clinical trials, ethics, pediatrics, and reproductive medicine. The NICHD IRB also supports the National Children's study.
NICHD clinical investigators contributed to over 90 publications in 2010. These include publications in Journal of Pediatrics, Lancet, American Journal of Obstetrics and Gynecology, Science Translational Medicine, Human Molecular Genetics, Proceedings of the National Academy of Sciences of the United States of America, Journal of Clinical Endocrinology and Metabolism, and the New England Journal of Medicine.
Contact
For more information, email fdporter@helix.nih.gov or visit http://science.nichd.nih.gov/confluence/display/ocd/Home.