You are here: Home > Microscopy and Imaging Core Facility
Microscopy and Imaging Core Facility

- James T. Russell, DVM, PhD, Director
- Louis (Chip) Dye, BS, Staff Scientist
- Vincent Schram, PhD, Staff Scientist
- Lynne A. Holtzclaw, BS, Senior Research Assistant (Biologist)
The mission of the NICHD Microscopy and Imaging Core (MIC) is to provide high-end light and electron microscopy services to all NICHD scientists. The Core is designed as a multi-user facility where investigators can, with a minimum of time and effort, prepare, image, and analyze their samples. Located in the fifth and sixth floors of Building 49, the facility is staffed by three full-time microscopists working under the supervision of James Russell: Vincent Schram oversees the light microscopy operations and IT infrastructure, Lynne Holtzclaw supports light microscopy, and immunohistochemistry, and Chip Dye manages the electron microscopy branch.
Mode of operation
The equipment and staff of the MIC are available to everyone within the Institute, with the proviso that all investigators receive equal support. The philosophy of the MIC is to ensure that only reliable, high-quality data are recorded on its instruments. For every new project, MIC staff meet with the Principal Investigator and the postdoctoral scientists involved in the study to discuss details of the experimental design. The background of the project and the imaging goals are discussed at that time, and the most appropriate techniques and instrumentation are determined. Users are asked to sign a document outlining the policies to follow when using the Core equipment. Positive feedback from long-term users suggests that this high level of interaction greatly improves the efficiency of each imaging project. The facility is accessible 24/7, and users can reserve time on each instrument by using an online calendar (next.cirklo.org/nichd).
The MIC has implemented a fee structure. Since October 2010, NICHD scientists who use MIC instruments for their imaging needs have been charged $20 to $30 per hour. Users from other Institutes will be charged at a slightly higher rate. The fee-for-service model for the core facility was approved by the Program Heads and PIs of NICHD through a comment process during the summer of 2010.
Light microscopy
The light microscopy branch of the MIC operates in four different areas:
Equipment and maintenance: The MIC operates several confocal laser scanning microscopes optimized for various applications:
- Zeiss LSM 510 inverted for high-resolution confocal imaging of fixed specimen
- Zeiss Live DuoScan for high-speed imaging of live cells
- Zeiss LSM 510 NLO for two-photon imaging of live tissue sections and live animals
- Perkin-Elmer spinning disk for low-light imaging of photo-sensitive specimens
- Dual-Channel Olympus Total Internal Reflection Fluorescence (TIRF) platform
- High-end conventional fluorescence microscope
- Zeiss Lumar Stereo Microscope
Live cell imaging is fully supported, with temperature control, heated perfusion, and solution change available on every microscope; most instruments also have an environmental chamber with temperature, CO2, and humidity control. Instrument downtime is kept to a minimum by providing full-time support to end users (phone and pager). For problems that require extensive repairs, all instruments are covered by a manufacturer's service contract and are usually serviced within the next business day.
User training and support: After counseling on specimen preparation and staining, each user receives hands-on training for the light microscope required by the project. The training covers the principles of fluorescence microscopy, confocal imaging, and optimum operation of the hardware platform. The initial training is followed by periodic refreshers at the user's request, or when MIC staff feel the equipment is not being used properly.
Image analysis: The MIC operates a data analysis center with three high-end workstations and imaging software (Metamorph, Volocity, Imaris, and Zeiss AIM). At the users' request, training and support are provided for each software package. When required, custom macros and high-throughput image analysis solutions are also provided. The facility also offers extensive data storage services with an enterprise-level file server and a data backup system. This infrastructure is used to safeguard images and move data from the facility to each user's location on campus.
Method development: During the past year, the MIC dedicated a significant amount of time and effort in the custom development of a Fluorescent Photo-Activatable Localization Microscopy (FPALM) platform. The image acquisition side of this instrument is now complete for red fluorescent proteins. With some initial funding, we have had a functional FPALM instrument for both green and red proteins since mid-spring 2010. FPALM also requires a complex image analysis module, which is still under development in collaboration with CIT, and will be available soon.
Upgrades: To ensure that it addresses users' needs optimally, the MIC continuously upgrades its equipment portfolio with new capabilities. During 2010 a new stereo microscope capable of imaging large specimens such as intact embryos was acquired from Zeiss Microsystems. This instrument incorporates fluorescence imaging and transmitted light imaging. Imaging is possible at different magnifications. The data analysis software has also been significantly upgraded during the past 12 months with the addition of Bitplane's Imaris software, which offers advanced three-dimensional rendering, quantitation, and filament tracing.
Electron microscopy
Because sample processing for electron microscopy (EM) is more sophisticated and requires close attention to detail, the EM component of MIC operates differently from the light microscopy projects.
Sample processing: Typically, all EM processing (fixation, embedding, cutting, and staining) is performed in-house by Mr. Dye. The MIC has a fully equipped EM laboratory with an LKB Pyramitome, a Leica CM3050-S Cryostat, and a Reichert Ultracut-E Ultramicrotome. Given to the labor involved, fewer projects are undertaken than for light microscopy. The PELCO Biowave Pro programmable incubator (Ted Pella, Inc.) has been instrumental in improving the quality of both ultrastructure and immunolabeling for immunohistochemistry by providing consistent and controlled incubation parameters.
Imaging: EM imaging is done most of the time by the microscopist except in cases where the user has the necess ary inclination and training. The MIC operates an aging JEOL 1010 transmission electron microscope, which is slated to be replaced during the Winter of 2010-2011 with a modern JEOL-1400. This upgrade will provide two new methodologies: cryo-electron microscopy and tomography imaging. The main advantage of cryo-EM is to preserve a high level of immunoreactivity and allows specimens to be imaged in a near native state. Cryo-EM will improve the quality of studies relying on immunogold labeling. Tomography permits imaging of three-dimensional structures at the EM level. This feature offers the ability to create 3D views of identified structures of interest that have been functionally characterized by other methods (for example, electrophysiology).
Method development: Mr. Dye has instituted techniques for EM-level immunohistochemistry and double immunolabeling to simultaneously mark two separate antigens. The use of specialized grids (LUXFilm) has also been developed. LUXFilm EM grids allow a view of the entire specimen and are crucial for imaging large structures, tracing features, searching for special details, and for tomography imaging. The MIC has also implemented a digital archive of all EM images and parameters, which will be available online to investigators in the near future.
Ancillary support
Given that NICHD's Division of Intramural Research laboratories are scattered throughout the NIH campus, the MIC provides all necessary techniques and facilities within a single building (Building 49): tissue culture hood, 5 and 10% CO2 incubators, animal holding and preparative space, vibratomes for live and fixed tissues. Lynne Holtzclaw provides outstanding technical expertise on advanced cell and tissue sample preparation for immunohistochemistry experiments.
Community outreach
The MIC is committed to promoting light and electron microscopy in the NICHD, DIR research community. Efforts are being made to educate investigators on the benefits and pitfalls of advanced imaging techniques. These initiatives include 1) coaching users on the principles of confocal microscopy, during training and via publication of comprehensive operating protocols for each microscope, 2) on-campus demonstrations of new instruments and software by vendors such as Zeiss, Olympus, Photometrics, Nikon and Perkin-Elmer, and 3) on-site assistance to investigators in their own laboratories operating their imaging equipment to optimize the quality of the data recorded. Furthermore, the MIC web site (mic.nichd.nih.gov) is an important resource for tutorials and protocols for both fixed and live cell microscopy.
In parallel with these efforts, the staff have continuous collaborations with other Institutes to promote the exchange of information and bring new imaging technologies to NICHD. Ongoing collaborations include imaging of live animals (Afonso Silva, NINDS) and sub-resolution light microscopy (F-PALM, trans-NIH Imaging initiative).
Facility usage
The MIC currently serves a total of 125 registered users associated with 41 NICHD PIs and 3 PIs of sister Institutes within the campus. During any given week, approximately 10 different users spend half a day or more on an MIC microscope. As of October 2010, users had logged more than 38,000 hours on the Core's equipment. Since its creation in 2004, usage has resulted in more than 78 publications, 7 of which have been co-authored by the Core personnel (see www.nichd.nih.gov/about/org/dir/other-facilities/cores/microscopyandimaging/publications for a complete list).
Looking ahead
The MIC continues to suffer from its aging equipment portfolio. Ninety percent of the light imaging platforms were purchased in 2004 and 2005 and no longer provide the level of performance required in today's competitive publishing environment. A proposal to replace the most used point-scanning confocal microscope was recently approved. The procurement process should begin at the start of fiscal year 2010-2011. After much delay, the existing 17-year-old JEOL transmission electron microscope is being replaced during the winter of 2010-2011 with a modern platform allowing for tomography and cryo-EM. MIC would still require additional equipment (high-pressure freezing unit, cryo-ultra-microtome, and a freeze substitution unit) in order to make CyroEM technology routinely available to investigators.
Efforts to develop sub-resolution fluorescence imaging (FPALM) were considerably slowed by the lack of funds to acquire necessary optical components. These are currently on order, and we eagerly await their arrival. Efforts towards developing multiple immunolabeling for EM are currently under way. The new JEOL 1400 instrument will significantly improve the EM capabilities of the Core by providing cryo-EM and tomography capabilities.
Although the MIC has been praised as a valuable resource to the NICHD community during its six years of existence, the facility is at a juncture at which its mission and support model within the Institute must be rethought. While the facility usage has been steadily increasing, it remains to be seen whether the transition to a fee-based mode of operation will maintain the level of use and provide adequate resources for sustainable operation and equipment upgrades. Continuing support from the Institute will be essential for the MIC to remain a viable operation and provide the NICHD community with the competitive edge that is so important in today's research environment.
Scientific activities within the MIC
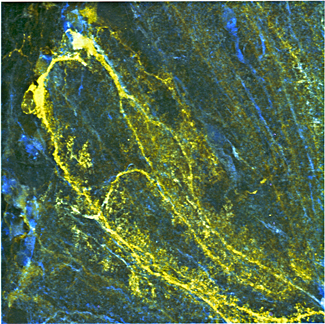
Bergmann glial cells in the cerebellum expressing the Cameleon calcium indicator YC 3.60
(click image to enlarge)
Calcium-based excitability in glial cells
Glial cells monitor and respond to neural activity by conditioning the extacellular milieu and by signaling within glial cell networks, as well as by sending signals back to neurons. Unlike neurons, which use electrical signals to communicate, glial cells possess a form of Ca2+-based excitability by which, in response to synaptic activity, they generate and propagate intracellular Ca2+ signals as waves over long distances. This intimate communication between glial cells and neurons is crucial for normal brain development and appears to play a critical role in the plastic functions of the brain. We aim to understand the nature of these signals in glial cells.
To this end, in collaboration with James Pickel, we generated transgenic mouse lines expressing YC3.60, a fluorescent Ca2+ indicator protein, which, by using the human S100β promoter sequence, was directed to be expressed only in astrocytes in the brain and in Schwann cells in peripheral nerves. We used two-photon confocal microscopy to image, in brain slice preparations, anesthetized intact mice, or isolated sciatic nerve preparations, cellular Ca2+ signals in astrocytes or Schwann cells either of which express the Ca2+ sensor transgene product YC3.60. We recorded glial cell signals evoked by exogenous application of neurotransmitter substances (glutamate) and electrical stimulation as well as spontaneous activity in tissue. Using a variety of known in situ and in vivo assays, we found that stimuli known to elicit Ca2+ signals in astrocytes caused substantial and rapid Ca2+ signals in the YC 3.60–expressing astrocytes. In addition, forepaw stimulation while imaging astrocytes through a cranial window in the somatosensory cortex in live mice revealed robust evoked and spontaneous Ca2+ signals.
In previous work, we showed that proteins involved in Ca2+ signaling are concentrated in regions of the Schwann cell around nodes of Ranvier in the sciatic nerve. The paranodal region and the juxtaparanode were rich in ion channels involved in metabotropic Ca2+ signaling. The finding suggested that specialized cellular regions may initiate Schwann cell signals during action potential propagation. In order to test this hypothesis, we imaged sciatic nerves isolated from transgenic mice expressing YC 3.60 in Schwann cells with two-photon confocal microscopy. We stimulated the nerve bundles with a suction electrode and recorded compound action potentials during stimulation. Action potentials elicited by electrical stimulation induced robust Ca2+ rises in Schwann cells, particularly in the paranodal region. This action potential–dependent Schwann cell Ca2+ response was abolished in the presence of purinergic antagonists that are specific for the P2Y2 subtype of receptor.
Application of exogenous purinergic agonists to isolated sciatic nerve axons readily elicited Ca2+ signals in Schwann cells, as revealed by YC 3.60 fluorescence changes. The rank order of potency of purinergic agonists was ATP>UTP>2MeSATP, suggesting the presence of P2Y-subtype purinergic receptors on Schwann cells. Furthermore, these purinergic agonist–elicited Ca2+ signals persisted in the complete absence of Ca2+ ions in the extracellular medium, suggesting that P2X-type purinergic receptors did not contribute to the signal. In support of this conclusion, prolonged exposure to UTP to deplete intracellular Ca2+ stores, while abolishing a response to ATP, did not evoke a response to a P2X-selective purinergic agonist. Our experiments showed that Schwann cells in situ express a functional p2Y-subtype of purinergic receptor. It is likely that this receptor system is involved in Schwann cell responses to acute nerve injury. It is well known that, following injury, Schwann cells retract, leading to initial demyelination, followed by regeneration.
The strategy of directing expression of Ca2+ indicator photoproteins in a cell-specific manner has proven extremely valuable for investigating glial cells' physiological responses during nervous system function, both in isolated preparations and in situ. Recently, by replacing the calcium sensor with the Ca2+-binding region of troponin-C of chicken muscle, Oliver Griesbeck of the Max Planck Institute in Germany designed a novel indicator protein called CerTn. While similar to calmodulin, the Ca2+-binding motif in chicken troponin-C does not bind to the calmodulin-binding proteins in the nervous system of mammals, thus making the CerTn series of indicators superior to YC3.60. We are now generating transgenic mice expressing TN-XXL, one of this class of indicators, in astrocytes, Schwann cells, and oligodendrocyte progenitors (OP cells). We plan to use the astrocyte-specific Glt-1 gene promoter to target to astrocytes and the cyclic nucleotide phosphodiesterase promoter to target to Schwann cells and OP cells. Once we have generated such transgenic mice, we should have the tools to investigate signaling in all three types of glial cells. We will also use cell-specific expression of cre-recombinase–containing constructs that target cell-specific promoters.
We initiated a collaborative study with Ranjan Gupta to investigate Schwann cell signals associated with chronic nerve compression injury. Gupta's laboratory has developed methods to produce such chronic injuries in mouse sciatic nerves. In the collaborative study, we will record Schwann cell Ca2+ signals at various times following injury in transgenic mice expressing YC 3.60 in Schwann cells. We hope to observe acute and chronic Schwann cell signals. We will attempt to block these signals using specific receptor antagonists, thereby hoping to ameliorate cellular pathology.special details, and for tomography imaging. The MIC has also implemented a digital archive of all EM images and parameters, which will be available online to investigators in the near future.
Publications
- Atkin SD, Patel S, Kocharyan A, Holtzclaw LA, Weerth SH, Schram V, Pickel J, Russell JT. Transgenic mice expressing a cameleon fluorescent Ca2+ indicator in astrocytes and Schwann cells allow study of glial cell Ca2+ signals in situ and in vivo. J Neurosci Meth. 2009;181:212-226.
- Balla T, Várnai P. Visualization of cellular phosphoinositide pools with GFP-fused protein-domains. Curr Protoc Cell Biol. 2009;24:24-44.
- Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, Hershfinkel M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J Neurosci. 2009;29:2890-2901.
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612-618.
- Koshimizu H, Senatorov V, Loh YP, Gozes I. Neuroprotective protein and carboxypeptidase E. J Mol Neurosci. 2009; 39:1-8.
- Park JJ, Koshimizu H, Loh YP. Biogenesis and transport of secretory granules to release site in neuroendocrine cells. J Mol Neurosci. 2009;37:151-159.
- Rafikova E, Melikov K, Ramos C, Dye L, Chernomordik L. Transmembrane protein-free membranes fuse into Xenopus nuclear envelope and promote assembly of functional pores. J Biol Chem. 2009;284:29847-29859.
- Tanaka N, Ito K, Stopfer M. Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. J Neurosci. 2009;29:8595-8603.
- Samoshkin S, Arnaoutov A, Jansen L, Ouspenski I, Dye L, Karpova T, McNally J, Dasso M, Cleveland D, Strunnikov A. Human condensin function is essential for centromeric chromatin assembly and proper sister kinetochore orientation. PLoS One. 2009;4:e6831.
- Uveges TE, Kozloff KM, Ty JM, Ledgard F, Raggio CL, Gronowicz G, Goldstein S, Marini JC. Alendronate treatment of Brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res. 2009;24:849-859.
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172-177.
- Russell JT. Imaging calcium signals in vivo: a powerful tool in physiology and pharmacology. Br J Pharmacol. 2010; in press.
Contact
For more information, visit mic.nichd.nih.gov.


