You are here: Home > Office of the Clinical Director
Office of the Clinical Director

- Forbes D Porter, MD, PhD, Clinical Director
- Meg Keil, PhD, Associate Director, Nursing and Protocol Navigation
- Maryellen Rechen, BS, RN, Special Assistant to the Clinical Director
- Linda Aspinall, Protocol Coordinator
- DuShon Hutchinson, Patient Care Coordinator
- Mellisa Goldstein, BA, Patient Care Coordinator
- Nghi Huynh, Patient Specimen Coordinator
Clinical Investigation Across the Lifespan
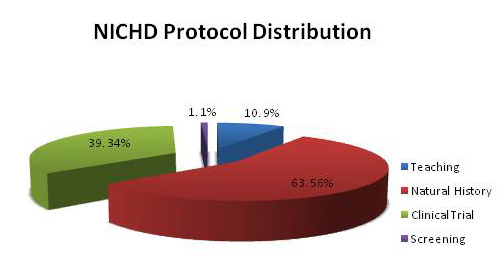
The NICHD intramural clinical research program currently includes 113 protocols with five main areas of focus: (i) adult, pediatric, and reproductive endocrinology, (ii) human genetics, (iii) normal growth and development, (iv) national/international public health, and (v) women's health. The protocols are conducted by 41 NICHD principal investigators and include natural history studies, teaching protocols, and therapeutic trials (Figure 1). In 2011, NICHD clinical protocols contributed to 136 publications and over 300 abstracts.
The conduct of studies is guided by two entities administered by the Office of the Clinical Director: the NICHD Institutional Review Board (IRB) and the NICHD Data Safety Monitoring Committee (DSMC). The NICHD IRB is chaired by Gilman Grave and has 15 members. The composition of the NICHD IRB is diverse, both in terms of medical and ethical expertise and affiliation. New IRB members have widened the board's expertise in reproductive endocrinology and the ethics of human subject research. The NICHD IRB supports the National Children's Study. The NICHD DSMC is chaired by Frank Pucino and has four additional members. Both committees possess expertise in issues related to clinical trials, ethics, pediatrics, and reproductive medicine.
Figure 1. NICHD clinical protocols
NICHD supports several types of clinical protocol including natural history/observational studies, screening protocols, teaching protocols, and therapeutic trials.