You are here: Home > Unit on Lung Injury and Repair
Lung Injury and Repair

- William J. Martin II, MD, Head, Unit on Lung Injury and Repair
- Ping M. Wang, PhD, Research Fellow
The Unit on Lung Injury and Repair focuses on improving our understanding of the pathogenesis of lung injury and repair so that novel therapeutic approaches can be developed to mitigate injurious events to the lung and facilitate more ordered repair that will preserve lung function and prevent evolution of disease.
Acute lung injury is characterized by alveolar epithelial cell injury and cell death. Bleomycin, a cancer chemotherapy agent, reproduces this pattern of injury by damaging both type I and type II alveolar epithelial cells. In a pattern similar to that observed in virtually in all forms of lung injury, an intense inflammatory and/or immunologic response accompanies the acute injury. Inflammatory and immune effector cells may modulate the lung injury and repair. Rapid repair of the acute injury likely represents the most effective means to dampen the intense inflammatory response.
The type II cell is the progenitor cell of the alveolar epithelium. The ability of the injured alveolar structure, the gas exchange unit of the lung, to recover depends on the alveolar cell restoring itself to health or being replaced in a timely fashion by another alveolar cell. To date, most therapeutic strategies in human subjects have focused on supportive care in the hope that endogenous mechanisms for repair would eventually predominate and that the alveolar structure and the lung would ultimately recover.
Alveolar tissue genesis and repair
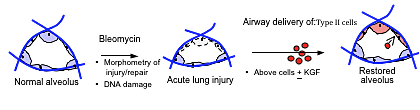
Figure 1. Alveolar tissue genesis
Recent reports suggest that bone-marrow derived stem cells can home to the lung during acute lung injury and trans-differentiate into alveolar epithelial cells, thereby offering a unique therapeutic window to repair the alveolar epithelium. More recently, the plasticity of the stem cells has been questioned, and cell fusion of the stem cell with a resident parenchymal cell may account for the findings. Stem cells are administered intravascularly with the expectation that some cells will migrate to and repopulate the injured areas. However, it is likely that some therapeutic stem cells will also be injured by the same mechanisms that injured the resident lung cells, thus limiting widespread recovery.
We chose to use a fundamentally different approach to cell-based repair of the injured alveolar epithelium. Our hypothesis was that airway delivery of donor type II alveolar epithelial cells to the lungs of mice with acute bleomycin-induced lung injury would result in engraftment and replacement of the injured alveolar epithelium, hasten recovery of lung architecture/function, and improve survival (Figure 1). First, we use airway delivery of progenitor cells to directly deliver large numbers of repair cells to the alveolar surface. Secondly, we use freshly isolated type II cells rather than bone marrow cells, as type II cells are the natural progenitor cells of the alveolar epithelium (Figure 2). Our data suggest that we can safely deliver, via the airway, large numbers of type II cells, which are almost immediately incorporated into the alveolar structures of bleomycin-injured mice and which subsequently differentiate into type I cells (Figure 3). Additionally, our data suggest that this approach to cell-based therapy may result in significant functional recovery from bleomycin-induced lung injury.
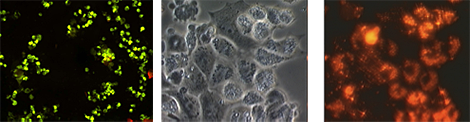
Figure 2. Labeling of isolated type II cells for in vivo use: left, anti-pro-SP-C & anti-T1-alpha; center, phase contrast (day 1 in culture); right, DiI-labeled cells
Cultured type II cells were incubated with 5 μM DiI for 30 min at 37°C. The cells were washed extensively in preparation for airway delivery. The left panel reveals that virtually all cells are positive for anti-pro-SP-C and negative for T1-alpha; the center panel shows a typical phase microscopy image of freshly isolated type II cells; the right panel shows the intense red-orange fluorescence of DiI labeling. DiI is retained by cells in vivo for months, and it has been suggested by others that Dil does not alter cell function or transfer from one cell to another.
Labeling of isolated type II cells for in vivo use: Our laboratory has isolated type II cells from both rats and mice for several years. Freshly isolated murine type II cells were verified as >95% pure by anti-pro-SP-C (FITC) and are negative for anti-T1-alpha (marker for type I cell). Fresh type II cells are incubated with the marker DiI, which is a fastidious label for cells and has been extensively used in our laboratory for in vivo tracking of the airway delivery of lung cells (Figure 2).
Airway delivery of type II cells: evidence of alveolar deposition: In the past, we used mechanical ventilation (MV) to assist in airway delivery of liposomes and microorganisms (Figure 3) (Wu et al., Proc Natl Acad Sci USA 2001; Zhang et al., Gene Ther 2003).
Future studies: We plan to complete studies of the effect of airway-delivered type II cells on bleomycin lung toxicity in mice in the near future. We will study type II cell engraftment, transition to type I cells, impact on injury and repair, and impact on survival. The use of stem cells to replace alveolar epithelial cells would offer a novel therapeutic approach to promote survival from severe acute lung injury.
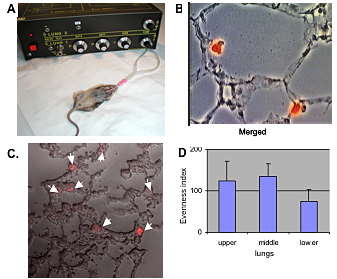
Figure 3. Airway delivery of type II cells: evidence of alveolar deposition
Murine type II cells were incubated with 5 μM DiI and were airway-delivered into the lungs of mechanically ventilated mice. A. Recipient mice; B. Fluorescence microscopy; C. confocal microscopy; both reveal that all DiI–donor type II cells are already associated with the alveolar wall in the first 24 h. We use alveolar macrophages (AMs) as control cells. Many AMs do not associate with the alveolar wall and are easily retrieved by brochoalveolar lavage (data not shown). D. To assess distribution, we counted DiI-labeled type II cells from several mice; data are expressed as an evenness index. Distribution is remarkably even.
Novel pathways for migration of inflammatory and immune cells into the lung during bleomycin toxicity
Inflammation of the lung is common to virtually all acute and chronic lung disorders. While inflammation is a natural host response to infections and injury, excessive or unresolved inflammation can play a role in cell injury and tissue remodeling with severe pathological consequences. Leukocytes such as neutrophils, eosinophils, and monocytes have been implicated as mediators of lung and vascular injury. Until now, leukocyte migration has been thought to occur at the level of the alveolar-caplillary unit.
We hypothesized that leukocytes directly enter the lung across larger vascular structures, which would account for the marked accumulation of leukocytes in the perivascular interstitium observed during acute inflammation in the lung. Using the bleomycin model, we provided clear evidence that leukocytes also directly migrate across the walls of larger arterioles and venules into adjacent perivascular interstitial areas after bleomycin-induced lung injury (1). This new observation in acute lung injury on the pathways by which leukocytes enter the lungs may help explain the associated perivascular pathology common to many acute and chronic lung disorders and may offer new therapeutic targets to modulate the inflammatory response in these disorders.
Publications
- Wang PM, Kachel DL, Cesta MF and WJ Martin II. Direct leukocyte migration across pulmonary arterioles and venules into the perivascular interstitium of murine lungs during bleomycin injury and repair. Am J Pathology 2011;178:2560-2572.
- Gwinn WM, Kapita MC, Wang PM, Cesta MF and WJ Martin II. Synthetic liposomes are protective from bleomycin-induced lung toxicity. Am J Physiol: Lung Cell Mol Phys 2011 301:L207-217.
- Martin II WJ, Glass RI, Balbus JM, FS Collins. Policy forum: A major environmental cause of death. Science 2011 334:180-181.
Collaborators
- Carl D. Bortner, PhD, Laboratory of Signal Transduction, NIEHS, Research Triangle Park, NC
- Sukhdev S. Brar, PhD, Laboratory of Molecular Genetics, NIEHS, Research Triangle Park, NC
- Mark F. Cesta, DVM, PhD, DVACP, Cellular and Molecular Pathology Branch, NIEHS, Research Triangle Park, NC
- William Gwinn, PhD, Respiratory Toxicology Group, NIEHS, Research Triangle Park, NC
- Diane L. Kachel, BA, University of Minnesota, Minneapolis, MN
- Mayanga C. Kapita, Duke University, Durham, NC
- Joel N. Meyer, PhD, Nicholas School of the Environment, Duke University, Durham, NC
- Bennett Van Houten, PhD, University of Pittsburgh Hillman Cancer Center, Pittsburgh, PA

