You are here: Home > Section on Molecular Neurobiology
Mechanisms Regulating Activity-Dependent Synaptic Plasticity and Gene Expression

- Andres Buonanno, PhD, Head, Section on Molecular Neurobiology
- Detlef Vullhorst, PhD, Staff Scientist
- Irina Karavanova, PhD, Biologist
- Robert Mitchell, PhD, Intramural Research Training Award Fellow
- Swagata Roychowdhury, PhD, Intramural Research Training Award Fellow
- Miguel Skirzewski, MS, Pre-Visiting Fellow
- Carolyn Keating, BS, Postbaccalaureate Fellow
- Katrina Furth, BS, Special Volunteer
A key feature in the etiology of several developmental psychiatric disorders such as schizophrenia, autism, and ADHD is the failure of cortical microcircuits to properly regulate the balance between excitation and inhibition. The balance is important to synchronize the firing pattern of local neuron ensembles. Such network activity, in particular oscillatory activity in the gamma-frequency range (30–80 Hz), is altered in psychiatric disorders, which may account for the cognitive and behavioral symptoms. We are interested in how Neuregulin and its receptor ErbB4, which are both genetically linked to psychiatric disorders, function in the developing brain to regulate synaptic plasticity, neuronal network activity, and behaviors that, in rodents, model features of psychiatric disorders. We identified a functional interaction between Neuregulin/ErbB4 and dopamine signaling in GABAergic interneurons that is critical for understanding how Neuregulin can regulate excitation/inhibition balance and synchronous activity in neuronal networks, given that both processes are important for the cognitive functions altered in psychiatric disorders.
To understand how Neuregulin-ErbB4 signaling regulates synaptic plasticity, neuronal network activity, and behaviors associated with psychiatric disorders, we are investigating how Neuregulin mediates its effects either indirectly by modulating excitation/inhibitory balance in neural circuits or directly by regulating the synaptic and intrinsic properties of ErbB4–expressing cells. To achieve these goals, we use a combination of techniques, including electrophysiological recordings in acute slices and dissociated cultures prepared from normal and genetically altered mice, reverse-microdialysis neurochemistry, confocal fluorescence microscopy in fixed and live tissue, proteomics analyses, and behavioral testing. The ultimate goal of this multi-disciplinary approach is to generate holistic models in which to investigate the developmental impact of genes that modulate excitatory/inhibitory balance and neuronal network activity and that consequently affect behaviors and cognitive functions altered in psychiatric disorders.
Importance of Neuregulin/ErbB4 signaling in parvalbumin-positive fast-spiking interneurons
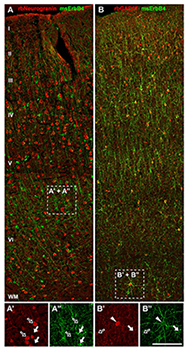
Figure 1. ErbB4 is expressed in a subset of GABAergic interneurons in monkey frontal cortex.
A, A’, A”: ErbB4 immunoreactivity (arrows) is absent from pyramidal cells labeled with rabbit anti-neurogranin antibody (open arrows). B, B’, B”: ErbB4 is strongly expressed in GAD-67–positive interneurons in layers II to VI, similar to rodent and human frontal cortex. Cells co-expressing both markers are labeled with arrowheads.
Using reverse-microdialysis neurochemistry and electrophysiology, we previously showed that Neuregulin 1 (NRG1) signaling via ErbB4 potently triggers dopamine release in the hippocampus and reverses long-term potentiation (LTP) at Schaeffer collateral-to-CA1 (SC-CA1) synapses by activating dopamine D4 receptors. Importantly, we found that ErbB4 is not detectable in excitatory pyramidal neurons but is abundantly expressed in GABAergic interneurons (Figure 1), particularly in fast-spiking parvalbumin-positive basket cells. Our observations strongly suggested that the effects of NRG/ErbB4 on glutamatergic synaptic plasticity are indirect. To address this question directly, we compared the effects of full vs. parvalbumin (PV) interneuron–specific Erbb4 ablation in genetically engineered mice (Figure 2). As expected, NRG–mediated inhibition of LTP induction and reversal of early-phase LTP were absent in full ErbB4 KO (knockout) mice. Interestingly, ErbB4 ablation in PV interneurons was sufficient to reproduce the effects of the full ErbB4 knockout. We also compared the two ErbB4 mutant mouse strains in a battery of behavioral tests relevant for psychiatric disorders and found that PV–restricted mutants replicated many, albeit not all, abnormalities observed in full ErbB4 mutant mice. The findings highlight the role of PV interneurons as a critical nexus of NRG/ErbB4 signaling and provide further evidence in support of a possible contribution of this pathway, genetically linked with increased risk for schizophrenia, to the pathophysiology underlying psychiatric disorders (1).
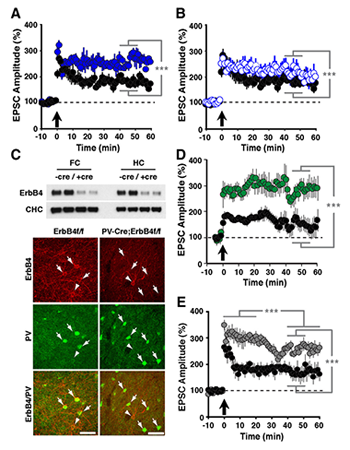
Figure 2. LTP in ErbB4– and NRG-1–mutant mice
Compared with normal controls (black circles in A, B, D, and E), slices from ErbB4−/− (A: solid blue circles) and ErbB4−/+ mice (B: open blue circles) exhibited enhanced LTP following pairing. C: Top, Western blot analysis of ErbB4 protein in whole-tissue lysates from frontal cortex (FC) and hippocampus (HC) of control ErbB4 f/f (−Cre) and PV-Cre;ErbB4 (+Cre) mice. Representative results from two animals are shown for each genotype. Bottom, co-immunofluorescence of ErbB4 (red) and PV (green) in the frontal cortex. In ErbB4 f/f controls, numerous PV+ cells coexpress ErbB4 (arrows). In contrast, ErbB4 is not detected in PV+ cells from PV–Cre;ErbB4 mice. Arrowheads mark ErbB4+ cells that are negative for PV. D: Selective ablation of ErbB4 in PV interneurons (green) results in higher LTP. E: LTP was also increased in slices from NRG-1+/− mice (grey).
NRG/ErbB4 and dopamine D4 receptor signaling converge in PV neurons and regulate gamma oscillations.
Click image to enlarge.
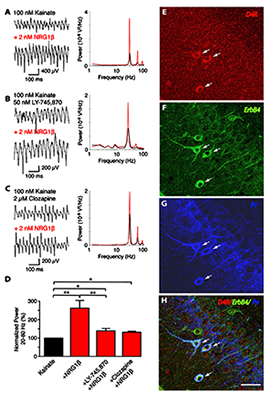
Figure 3. Convergence of ErbB4 and D4R signaling in PV–positive interneurons
Representative sample traces (left) and power spectra (right) of kainate-induced gamma oscillations in rat slices. NRG-1b (2 nM) was tested in combination with (A) kainate alone (n=8), (B) kainate and L-745,870 (50 nM; n=8), or (C) kainate and clozapine (2 µM; n=5). D: Summary analysis of normalized data from (A–C). Values for kainate treatment are set as 100%. E–H: Representative images of triple immunofluorescence histochemistry in hippocampal CA3. Overlay images showing PV+ neurons that also co-express D4 and ErbB4 receptors (arrows).
We previously reported that NRG/ErbB4 signaling modulates the power of kainate-induced hippocampal gamma oscillations (Figure 3). Because NRG1 dramatically raises extracellular dopamine levels in the hippocampus, we investigated the relationship between NRG/ErbB and dopamine signaling in hippocampal gamma oscillations. In collaboration with André Fisahn’s lab, we used selective agonists for different dopamine G-coupled receptors that elevate (D1 and D5 receptors) or reduce (D2, D3, and D4 receptors) the synthesis of cAMP, and found that only an agonist specific for D4 receptors (PD168077) augmented the power of gamma oscillations. By contrast, agonists for D1/D5 and D2R/D3 receptors were without effect. The rise in gamma oscillation power induced by PD168077 was totally blocked by a highly specific D4 receptor antagonist (L-745,870), further stressing the importance of this receptor for neuronal network activity. Importantly, we found that L-745,870 and the antipsychotic clozapine, which preferentially target D4 receptors, also blocked increases in gamma oscillation power by NRG1. Using double in situ hybridization (in collaboration with Ursula Winzer-Serhan) and immunofluorescence histochemistry, we showed that hippocampal D4 receptor mRNA and protein are highest in a subset of GABAergic interneurons. Importantly, D4 and ErbB4 receptors are coexpressed in PV–positive fast-spiking basket cells, a type of somatic-targeting GABAergic interneuron that is critical for regulating gamma oscillations. This novel cross-talk between D4 and ErbB4 receptor signaling to augment gamma oscillation power and their coexpression in PV–positive interneurons suggest a cellular mechanism that may be compromised in various psychiatric disorders affecting cognitive control (2).
Neuregulin directly decreases voltage-gated sodium currents in hippocampal ErbB4–expresssing interneurons.
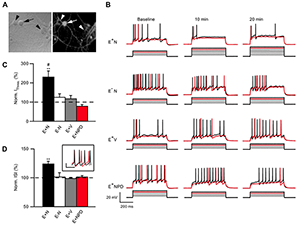
Figure 4. NRG1 reduces ErbB4 neuron excitability.
A: Neurons live-labeled with an ErbB4 antibody (arrow: ErbB4+; arrowhead: ErbB4−). B: Representative traces of current injection steps in ErbB4+ or ErbB4− neurons treated with NRG1 (E+N and E−N), vehicle-treated ErbB4+ neurons (E+V) and ErbB4+ neurons treated with NRG1 and the ErbB receptor inhibitor PD158780 (E+NPD). The rheobase current is shown in red and one subsequent trace is shown in black. Current was injected in 50 pA steps. C: Summary graph of normalized current threshold to evoke an action potential (AP) at 20 min. D: Summary graph of normalized inter-spike interval (ISI) at 20 min averaged over all current. Inset shows example of an ErbB4 cell firing at baseline (black) and 20 min (red) after NRG1 application at 700 pA injection.
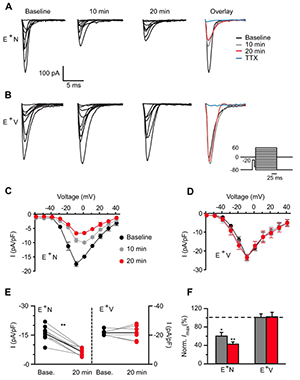
One of our primary goals has been to investigate if and how NRG1 may directly affect the intrinsic properties of ErbB4–positive (ErbB4+) interneurons. To this end, we resorted to dissociated hippocampal cultures, which are devoid of all hippocampal-projecting afferent connections, and to blocking all synaptic activity pharmacologically. We initially analyzed the effects of NRG1 on the excitability of ErbB4+ and ErbB4− neurons, identified in live labeling by an ectodomain-targeting antibody, using whole-cell current-clamp recordings. We found that NRG1 reduced firing of ErbB4+ but not of ErbB4− neurons by shifting the action potential threshold (Figure 4). Using voltage-clamp recordings, we determined that the effects are primarily attributable to decreased voltage-gated sodium channel activity, as current density was attenuated by about 60% after 20 minutes of NRG1 treatment (Figure 5). This is the first study to identify direct actions of NRG1 on voltage-gated sodium channel function in ErbB4–expressing interneurons, thereby offering novel insights into how NRG1/ErbB4 signaling can directly impact GABAergic interneuron activity and potentially affect excitation/inhibition (E/I) balance (3).
Figure 5. NRG1 application dramatically reduces voltage-gated sodium channel currents.
A, B: Representative traces of voltage-gated sodium channel (Nav) currents from an ErbB4 interneuron before (baseline) and after NRG1 (A) or vehicle (B) application for 10 and 20 min. Far right shows overlaid maximum Na currents. Inset shows voltage-clamp protocol used to evoke somatic Na channel current. C, D: Averaged I/V relationship for Nav activation showing the NRG1–specific decrease in Nav density (C) compared with vehicle (D). E: Individual Nav current responses to NRG1 (E+N, left) or vehicle application (E+V, right) at 20 min are plotted with their own baseline (gray lines). Average response depicted with black line. F: Averaged normalized maximum current density from E+N and E+V cells.
NRG1 signaling directly influences AMPA receptor (AMPAR) activity in glutamatergic ErbB4–expressing cerebellar granule neurons.
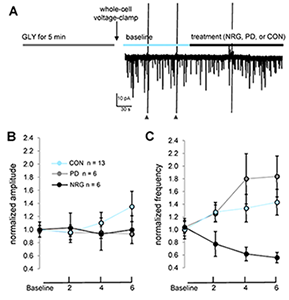
Previous studies demonstrated how NRG1 indirectly affects plasticity at glutamatergic synapses in principal glutamatergic neurons in the hippocampus and frontal cortex. In collaboration with Catherine Fenster, we analyzed the effects of NRG1 on developing cultured cerebellar granule cells (CGCs). The cultures consist predominantly of granule neurons that express ErbB4. We found that NRG1 does not affect whole-cell AMPAR– or NMDAR–mediated currents nor the frequency or amplitude of spontaneous NMDAR– or AMPAR–mediated miniature excitatory post-synaptic currents, in baseline conditions of CGCs grown for 10–12 days in vitro. However, we found that high glycine induces a form of chemical potentiation (chemLTP) in CGCs characterized by an increase in AMPAR-mEPSC frequency that is reduced by NRG1 treatment (Figure 6). Because, under our culture conditions, CGCs express very low levels of the AMPAR GluA1 subunit but high levels of GluA4, our data suggest that the NRG1 effect may be mediated by GluA4 subunits. The study shows, for the first time, that high glycine can induce plasticity at glutamatergic synapses in CGCs and that acute NRG1/ErbB signaling can regulate glutamatergic plasticity in CGCs. Taken together with our previous studies, our results suggest that, similar to SC-CA1 synapses, NRG1 effects on CGCs are activity-dependent and mediated by modulation of synaptic AMPARs (4).
Figure 6. NRG1 reduces AMPAR–mEPSC frequency, but not amplitude, following chemLTP.
A: Experimental protocol used to assess effects of treatments following high glycine. Cells were immediately voltage-clamped following exposure to high glycine, and baseline data were collected for 4–6 min prior to and following treatment onset with either PD158780, NRG1, or vehicle control. Cells were continuously perfused with extracellular fluid (ECF) containing 1 mM MgCl2, 500 nM tetrodotoxin, and 50 µM bicuculline. Data points plot mean AMPA–mEPSC amplitude (B) and frequency (C) at baseline and at indicated time points following treatments. **P < 0.001 (repeated measures ANOVA, time × treatment interaction).
Functional analysis of the extrasynaptic ErbB4 receptor proteome using a proteomics approach
In collaboration with Sanford Markey's group, our lab is using a combination of biochemical and immunological approaches to identify novel ErbB4–interacting proteins. We focused on identifying proteins that interact with extrasynaptic ErbB4 receptors, a pool that constitutes approximately 80–90% of the total receptor population in cortical and hippocampal interneurons and which has mostly been unstudied. Using a novel rabbit monoclonal ErbB4 antibody (mAb10) generated and characterized in our lab (Vullhorst et al. J Neurosci 2009;29:12255), in combination with immunoprecipitation (IP) from metabolically active cortical synaptosome preparations prepared from a large number of brain cortices, we employed liquid chromatography–tandem mass spectrometry (LC/MS/MS)–based proteomics to determine the proteome composition of ErbB4 complexes specifically immunoprecipitated (IPed) by mAb10. The specificity of this approach was confirmed by comparing the proteins identified in IPs performed from wild-type with those from ErbB4 knockout mice. Among the receptors, signaling proteins, and cytoskeletal components, IPed selectively by mAb10 and characterized by LC/MS/MS (including ErbB4), we identified the GABAA receptor α1 subunit (see below).
ErbB4 reduces synaptic GABAA currents independently of its receptor tyrosine kinase activity.
Using the aforementioned unbiased approach of ErbB4 IP combined with LC/MS/MS, we identified a novel and clinically relevant ErbB4–interacting protein: the GABAA receptor α1 subunit (GABAR α1). Co-IP and double-immunofluorescence experiments using GABAR α1– and ErbB4–specific antibodies indicate that both proteins interact in GABAergic interneurons in vivo and in dissociated cultures. Using a combination of electrophysiological, biochemical, and cell-biological techniques, we identified a novel NRG–mediated ErbB4 signaling pathway that couples ErbB4 to reduced postsynaptic GABAR currents onto inhibitory interneurons.
Remarkably, we found that this novel ErbB4 signaling pathway, which reduces postsynaptic GABAR currents on GABAergic interneurons, acts independently of ErbB4’s canonical receptor tyrosine kinase activity. We demonstrate inhibition of ErbB4's tyrosine kinase activity by the selective antagonist PD158780, which, as we previously demonstrated, totally blocks the effects of NRG on plasticity and sodium channel currents, but in this case fails to block the effects of NRG on reducing GABAR α1 receptor–mediated currents. Cultured neurons prepared from ErbB4 KO mice do not down-regulate GABAR α1 receptor–mediated currents in response to NRG, but can do so if rescued by infection with AAV viruses expressing ErbB4 harboring a point mutation that inactivates its canonical tyrosine kinase activity. While the effects of NRG on GABAR α1 internalization do not require ErbB4 receptor tyrosine kinase activity, both clathrin-mediated endocytosis and protein kinase C are necessary to reduce GABAR inhibitory currents and synaptic alpha 1. Our results reveal a new function of ErbB4, independent of its tyrosine kinase activity, which modulates postsynaptic inhibitory control of hippocampal interneurons and may provide a novel pharmacological target in the treatment of neuropsychiatric disorders and epilepsy (5).
Publications
- Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ, Buonanno A. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci 2012;32:2988-2897.
- Andersson RH, Johnston A, Herman PA, Winzer-Serhan UH, Karavanova I, Vullhorst D, Fisahn A, Buonanno A. Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc Natl Acad Sci USA 2012;109:13118-13123.
- Janssen-Schroeder MJ, Leiva Salcedo DE, Buonanno A. Neuregulin directly increases voltage-gated sodium currents in hippocampal ErbB4-expressing interneurons. J Neurosci 2012;32:13889-13895.
- Fenster C, Vullhorst D, Buonanno A. Acute neuregulin-1 signaling influences AMPA receptor mediated responses in cultured cerebellar granule neurons. Brain Res Bull 2012;87:21-29.
- Mitchell RM, Janssen MF, Karavanova I, Vullhorst D, Furth K, Makuskey A, Markey S, Buonanno A. ErbB4 receptor reduces synaptic GABAA currents independently of its tyrosine kinase activity. Proc Natl Acad Sci USA 2013;in press.
Collaborators
- Shashank Dravid, PhD, Creighton University, Omaha, NE
- Catherine Fenster, PhD, The College of Wooster, Wooster, OH
- André Fisahn, PhD, Karolinska Institute, Stockholm, Sweden
- Kenneth Fish, PhD, University of Pittsburgh, Pittsburgh, PA
- Luis Hernández, MD, Universidad de los Andes, Mérida, Venezuela
- David A. Lewis, MD, University of Pittsburgh, Pittsburgh, PA
- John E. Lisman, PhD, Brandeis University, Waltham, MA
- Sanford P. Markey, PhD, Laboratory of Neurotoxicology, NIMH, Bethesda, MD
- Christopher McBain, PhD, Program on Developmental Neuroscience, NICHD, Bethesda, MD
- Jörg Neddens, PhD, JSW Life Sciences, Grambach, Austria
- Alon Shamir, PhD, Mazra Mental Health Center, Akko, Israel
- Ludovic Tricore, PhD, Program on Developmental Neuroscience, NICHD, Bethesda, MD
- Ursula H. Winzer-Serhan, PhD, Texas A&M University, College Station, TX
Contact
For more information, email buonanno@mail.nih.gov or visit smn.nichd.nih.gov.