You are here: Home > Section on Neurophysiology and Biophysics
Structural Biology of the Glutamate Receptor

- Mark L. Mayer, PhD, Head, Section on Neurophysiology and Biophysics
- Sagar Chittori, PhD, Visiting Fellow
- Janesh Kumar, PhD, Visiting Fellow
- Suvendu Lomash, PhD, Visiting Fellow
- Carla Glasser, PhD, Technical Specialist
- Robert Alberstein, BS, Postbaccalaureate Fellow
- Alecia Thomas, BS, Postbaccalaureate Fellow
Ionotropic glutamate receptors (iGluRs) are membrane proteins that act as molecular pores permeable to sodium and calcium ions. At the majority of excitatory synapses in the mammalian nervous system, iGluRs mediate rapid signal transmission, converting the pre-synaptic action potential–triggered release of glutamate into a depolarizing post-synaptic potential. The seven iGluR gene families in humans encode 18 subunits, which assemble to form three major functional families named after the ligands that were first used to identify iGluR subtypes in the late 1970s: AMPA, kainate, and NMDA. Given the essential role of iGluRs in normal brain function and development, and mounting evidence that dysfunction of iGluR activity mediates several neurological and psychiatric diseases and damage during stroke, we devote substantial effort to analyzing iGluR function at the molecular level. Atomic resolution structures solved by protein crystallization and X-ray diffraction provide a framework for designing electro-physiological and biochemical experiments aimed at defining the mechanisms underlying ligand recognition, the gating of ion channel activity, and the action of allosteric modulators. Information derived from these experiments will permit the development of subtype-selective antagonists and allosteric modulators with novel therapeutic applications and reveal the inner workings of a complicated protein machine that plays a critical role in brain function.
Key issues in the field include obtaining structures for iGluRs trapped in their three major conformational states, i.e., the resting, activated, and desensitized states, and obtaining insight into the energy landscapes connecting these states. Also of interest are the evolutionary relationships connecting iGluRs in different species and how structurally related chemosensors bind to a wide range of small molecules.
Expression, purification, and structural analysis of full-length kainate receptor ion channels
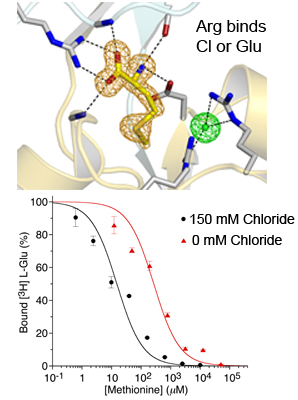
Figure 1. Anions mediate binding of hydrophobic amino acids to the glutamate receptor from A. vaga.
The upper panel shows an electron density omit map for methionine bound to AvGluR1 together with density for a chloride ion that acts as a counter charge for the Arg side chain interacting with bound glutamate and aspartate. The lower panel shows chloride dependence for a displacement experiment with binding of methionine to the purified AvGluR1 LBD with either 0 or 150 mM extracellular chloride.
Structural studies on CNS membrane proteins are notoriously difficult because commonly used bacterial protein expression systems cannot be employed to express eukaryotic membrane proteins. We succeeded in obtaining highly purified preparations of two different kainate receptor subtypes expressed in both insect cells, using baculovirus expression, and in HEK 293 cells, using transient transfection. For both systems, cultures must be grown on the 12-liter and larger scale in order to obtain sufficient protein for biochemical and structural analysis. Because the amounts of protein obtained are still at least an order of magnitude lower than for soluble proteins, it is necessary to use highly efficient methods for establishing conditions in which the proteins remain, for several days following purification, correctly folded and do not aggregate or dissociate into dimers and monomers. To do this, we use tryptophan fluorescence size exclusion chromatography (FSEC), for which only microgram quantities of purified protein are required for each run of a 300 mM–length analytical gel filtration column. Using this approach, we screened numerous detergents, lipids, receptor ligands, and mutants with selective removal of glycosylation sites and various C-terminal deletions. Crystallization trials using conventional detergent-solubilized protein, as well as bicelles and lipidic cubic phases, are under way, with initial hits of microcrystals that are candidates for optimization. The proteins we prepared using this approach were also used successfully to solve 25 Å resolution structures for GluK2 in resting and desensitized states by cryo-electrom tomography. In ongoing work single particle analysis is being used to obtain structures at much higher resolution, and in the current maps well ordered alpha helices are clearly visible. This work is being performed in collaboration with Sriram Subramaniam and his graduate student Joel Meyerson.
Structural studies on the amino-terminal domain of iGluRs
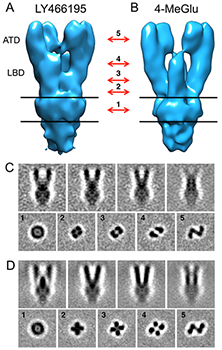
Figure 2. Electron density maps for GluK2 in resting and desensitized states
A, B. Isosurface representations of GluK2 density maps in the resting (A) and desensitized (B) states obtained by cryo-electron tomography combined with sub-volume averaging. Absolute density representations, shown as slices through different sections of the tomograms in C and D, clearly reveal differences between the two conformations; in each panel, the images in the upper row show four projections of the front view of the maps shown in panels A and B, spaced by successive rotations around the long axis by 15°; the bottom rows show five sections through the maps perpendicular to the long axis, at the locations indicated by the red arrows between panels A and B; the numbers indicated with the arrows correspond to the sections shown in panels C and D, with approximate locations as follows: 1, middle of the transmembrane region; 2, membrane-proximal region on the extracellular side; 3, lower lobe of the ligand-binding domain (LBD); 4, upper lobe of the LBD; 5, central region of the ATD. Structures were solved by the Subramaniam lab.
The iGluRs are excitatory neurotransmitter receptors with a unique molecular architecture, in which the extracellular domains assemble as a dimer of dimers. The structure of individual dimer assemblies was established previously for both the isolated ligand-binding domain (LBD) and more recently for the larger amino-terminal domain (ATD). How these dimers pack to form tetrameric assemblies in intact iGluRs has remained controversial. Adding to the complexity, native glutamate receptor ion channels are tetrameric assemblies containing two or more distinct subunits. While some AMPA and kainate receptors can form functional homomeric ion channels, the KA1 and KA2 subunits are obligate heteromers, which function only in combination with GluR5-7; in the brain the major kainate receptor species contains both GluR6 and KA2 subunits in unknown stoichiometry and geometric arrangement. The mechanisms controlling glutamate receptor assembly are believed to involve an initial step in which the ATDs assemble as dimers, but how the GluR6 and KA2 subunits cooperate to do this had not been established. Using sedimentation velocity analysis, we found that the ATDs of GluR6 and KA2 coassemble as a heterodimer of Kd 11 nM, which is 32,000-fold lower than the Kd for homodimer formation by KA2. We then solved crystal structures for the GluR6/KA2 ATD heterodimer and heterotetramer assemblies. Using these structures as a guide, we performed a mutant cycle analysis to probe the energetics of assembly and showed that high-affinity ATD interactions are required for biosynthesis of functional heteromeric receptors. The high affinity of the KA2 subunit for GluR6 ensures that ATD heterodimers form early during the process of biogenesis, before trafficking comes into play, and in addition provides a mechanism by which formation of functional GluR6 homotetramers that lack the KA2 subunit is suppressed, while ensuring a 2:2 stoichiometry of assembly. The binding mechanism generating the kainate receptor heterodimer assembly involves residues present in both the R1 and R2 lobes of KA2 protomers.
Molecular biophysical studies on AMPA receptor dimer assembly
Sedimentation velocity (SV) analytical ultracentrifugation (AUC) has re-emerged as an important tool for the characterization of biological macromolecules and has been used extensively for analysis of monomer-dimer and dimer-tetramer equilibria for the ATDs and LBDs of iGluRs. Prior reports on AMPA receptor GluA2 dimerization differed in their estimate of the monomer-dimer Kd by a 2,400-fold range, with no consensus as to whether the ATD forms tetramers in solution. In pilot sedimentation velocity (SV) experiments, performed using absorbance and fluorescence detection, we found a narrow range of monomer-dimer Kd values in the low nM range for GluA2, with no detectable formation of tetramers, and no effect of glycosylation or the polypeptide linker connecting the ATDs and LBDs; for GluA3 the monomer-dimer Kd was 5.6 µM, again with no detectable tetramer formation. SV experiments with fluorescence detection for GluA2 labeled with 5,6-carboxyfluorescein (FAM), and fluorescence anisotropy measurements for GluA2 labeled with DyLight405, yielded comparable Kd values; however, the sedimentation coefficients measured by AUC using absorbance differed from those obtained using fluorescence systems (FDS). Given the unique capabilities of the fluorescence detection AUC for future experiments, we conducted an extensive series of experiments to characterize this system, with the goal of identifying procedures that permit accurate Kd measurements at nM protein concentrations. We found by FDS-SV that the FAM label produces a mixed population of receptors with artificially high and low affinity, while DL-488 labeled protein, and a GluA2-EGFP fusion protein were monodisperse, and had identical Kd values. By performing FRET experiments on GluA2 separately labeled with donor and acceptor dyes, and the kinetics of dimer dissociation measured by the kinetics of quenching following addition of excess unlabeled protein, we obtained independent evidence for perturbation of dimer stability by the FAM label. Thus, in order to take advantage of FDS-SV experiments, control experiments to test for artifacts produced by fluorescent labels are an important consideration. The work is being performed in collaboration with Joy Zhao and Peter Schuck.
Structural studies on glutamate receptors from primitive organisms
We expressed the LBD of a recently discovered glutamate receptor from the primitive eukaryote Adineta vaga (AvGluR1) in Origami B (DE3) and purified it to homogeneity. The apo form of AvaR1 was generated for a direct filter-binding assay, yielding a Kd of 200 nM. Competition assays reveal that AvGluR1 has promiscuous ligand-binding activity, and in addition to recognizing acidic and neutral amino acids, binds also to alanine, methionine, and phenylalanine, but not to amino acids with a beta carbon branch, such as valine and threonine. Electrophysiological assays establish that, like glutamate and aspartate, alanine, methionine, and phenylalanine are efficient agonists. Crystal structures solved to investigate this unusual binding pattern reveal that the gamma COOH group of glutamate and aspartate is coordinated by by a pair of arginine residues in domain 2, unlike in any other iGluR LBD structure solved to date, but similar to the mechanisms for binding of glutamate by type III GPCRs. Neutral and hydrophobic amino acids use a chloride ion as a surrogate ligand, compensating for the missing ligand carboxylate group. Consistent with this, competition assays with tritiated glutamate reveal that the binding of alanine and serine, but not of glutamate and aspartate, to AvGluR1 is chloride dependent.
Structural basis for allosteric modulation of kainate receptors by zinc
Kainate receptors (KARs) play a key role in the regulation of synaptic networks. We find that zinc, a cation released at a subset of glutamatergic synapses, potentiates glutamate currents mediated by homomeric and heteromeric KARs containing GluK3 at 10–100 µM concentrations, whereas zinc inhibits other KAR subtypes. Potentiation of GluK3 currents is mainly attributable to reduced desensitization, as shown by kinetic analysis and modeling. Mutation and crystallographic analyses revealed that a specific zinc-binding site is formed at the base of the LBD dimer interface by a GluK3–specific aspartate (Asp759), together with two conserved residues, His762 and Asp730, the latter located on the partner subunit. This site of interaction identifies the LBD as a hot spot for allosteric modulation, given that kainate receptors are also modulated by sodium and chloride ions, which bind to discrete sites at the top of the dimer assembly, while AMPA–receptor allosteric modulators bind in a crevice between subunits in their LBD dimer assembly. Our analysis suggests that tetrameric GluK2/3 receptors are likely assembled as pairs of heterodimeric LBDs. Therefore, zinc binding stabilizes the labile GluK3 dimer interface, slows desensitization, and potentiates currents, providing a new mechanism for KAR potentiation at glutamatergic synapses.
Additional Funding
- NICHD IRP
Publications
- Schauder DM, Kuybeda O, Zhang J, Klymko K, Bartesaghi A, Borgnia MJ, Mayer ML, Subramaniam S. Glutamate receptor desensitization is mediated by changes in quaternary structure of the ligand binding domain. Proc Natl Acad Sci USA 2013;110:5921-5926.
- Lomash S, Chittori S, Brown P, Mayer ML. Anions mediate ligand binding in Adineta vaga glutamate receptor ion channels. Structure 2013;21:414-425.
- Yao Y, Belcher J, Berger AJ, Mayer ML, Lau AY. Conformational analysis of NMDA receptor GluN1, GluN2, and GluN3 ligand-binding domains reveals subtype-specific characteristics. Structure 2013;Epub ahead of print.
- Veran J, Kumar J, Pinheiro PS, Athané A, Mayer ML, Perrais D, Mulle C. Zinc potentiates GluK3 glutamate receptor function by stabilizing the ligand binding domain dimer interface. Neuron 2012;76:565-578.
- Zhao H, Berger AJ, Brown PH, Kumar J, Balbo A, May CA, Casillas E Jr, Laue TM, Patterson GH, Mayer ML, Schuck P. Analysis of high-affinity assembly for AMPA receptor amino-terminal domains. J Gen Physiol 2012;139:371-388.
Collaborators
- Albert Lau, PhD, The Johns Hopkins University School of Medicine, Baltimore, MD
- Christophe Mulle, PhD, Interdisciplinary Institute for Neuroscience, Université de Bordeaux, Bordeaux, France
- Peter Schuck, PhD, Laboratory of Cellular Imaging and Macromolecular Biophysics, NIBIB, Bethesda, MD
- Sriram Subramaniam, PhD, Laboratory of Cell Biology, Center for Cancer Research, NCI, Bethesda, MD
- Joy Zhao, PhD, Laboratory of Cellular Imaging and Macromolecular Biophysics, NIBIB, Bethesda, MD