Signaling and Secretion in Neuroendocrine Cells
- Stanko S. Stojilkovic,
PhD, Head, Section on Cellular Signaling - Stephanie Constantin, PhD, Staff Scientist
- Naseratun Nessa, PhD, Visiting Fellow
- Sonja Sivcev, PhD, Visiting Fellow
- Kosara Smiljanic, PhD, Visiting Fellow

The Section was formed in 1993 to investigate cell-signaling cascades, gene expression, and secretion in hypothalamic and pituitary cells, with special emphasis on interactions between electrical events at the plasma membrane and receptor-controlled pathways. Specifically, we address how these neuroendocrine cells use ion channels and G protein–coupled receptors (GPCRs) as signaling platforms for efficient information processing. For this purpose, we characterize both native and recombinant receptors and channels that have been cloned from neuroendocrine cells. In the past, our work focused on the role of inositol-trisphosphate receptors in oscillatory calcium release of pituitary cells, the mechanism of periodic activation of these channels, and the complex way of synchronizing calcium release from intracellular stores and the electrical activity of cells. We also characterized the voltage-gated channels expressed in neuroendocrine cells, the cell type–specific patterns of electrical activity and channels involved, the physiological relevance of such activity, and the crosstalk between GPCRs and ion channels. More recently, we characterized ligand-gated receptor channels expressed in pituitary cells, including ATP–gated P2X receptor channels. Our current work focuses on age-, sex-, and tissue structure–specific pituitary signaling, transcription, and secretion, the heterogeneity of pituitary secretory cells reflecting their postnatal genesis, and cell type–specific exocytic pathways. Ongoing and proposed projects include the use of transgenic and conditional knockout mouse models. The results already obtained by these projects and the results of other research have revealed the enormous complexity and physiological relevance of the intracellular signaling system in the pituitary gland and the need for additional investigations. The information we are currently obtaining and the research planned should continue to be useful to investigators and clinicians in the neuroendocrine field, as well as to the wider scientific community.
What is known and unknown about the role of the neuroendocrine genes Ptprn and Ptprn2
We continue to investigate genes expressed in mammalian hypothalamic and pituitary cells and their role in cell signaling and function. Our initial studies showed that simultaneous knockout of the neuroendocrine marker genes Ptprn and Ptprn2 causes infertility in female but not in male mice. To elucidate the mechanism of the sex-specific roles of Ptprn and Ptprn2 in mouse reproduction, we analyzed the effects of their double knockout (DKO) on the hypothalamic-pituitary-gonadal axis during postnatal development. Our research revealed that the hypothalamus is the primary component of the reproductive axis affected by DKO. While only two major populations of hypothalamic neurons have been examined, and others cannot be ruled out, these central defects are sufficient to establish a hypothesis explaining female infertility. Reduced expression of the gene that encodes kisspeptin (Kiss1) and kisspeptin synthesis in the arcuate nucleus of DKO animals reduces Gnrh1 expression and pulsatile gonadotropin-releasing hormone (GnRH) secretion. This in turn reduces the expression of the gonadotroph-specific genes Lhb, Fshb, and Gnrhr in females and males. While Gnrhr gene expression is reduced, sufficient GnRH receptors remain to drive calcium signaling and luteinizing-hormone secretion, as well spermatogenesis and oogenesis. However, reduced kisspeptin synthesis in the RP3V region (rostral periventricular area of the third ventricle) of the hypothalamus affects the burst release of GnRH required for ovulation and postovulatory steroidogenesis, leading to female infertility [Reference 1].
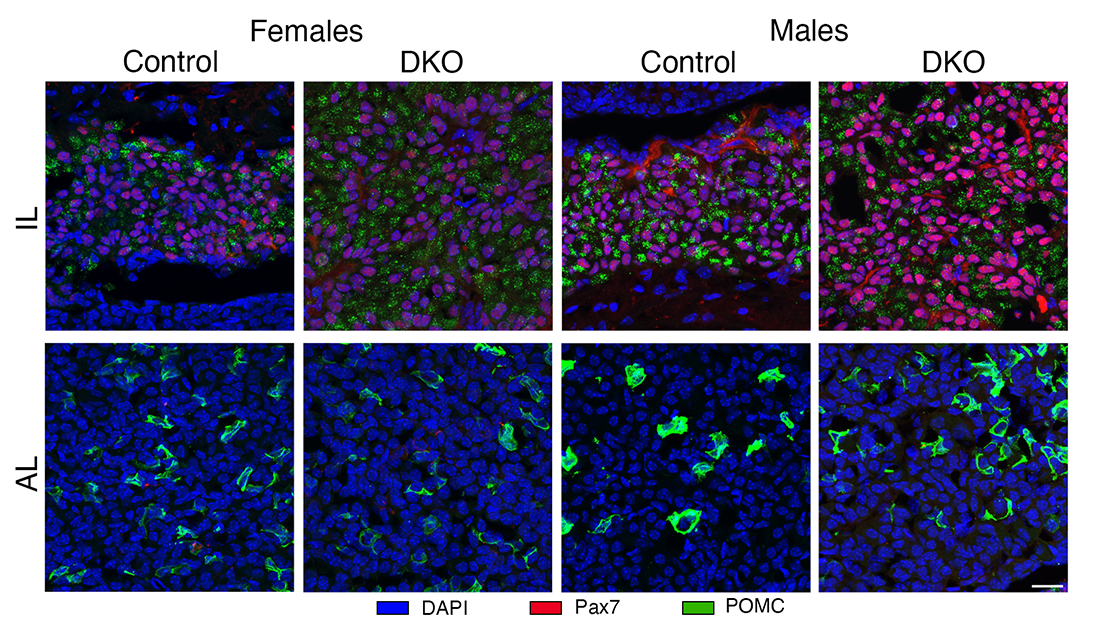
In a recent study, we examined the roles of Ptprn and Ptprn2 on corticotroph and melanotroph functions during development. In both sexes, DKO induced an increase in the expression of the corticotroph and melanotroph genes Pomc and Tbx19 and the melanotroph-specific gene Pax7. We also found, both in vivo and in vitro, increased synthesis and release of beta-endorphin, alpha-melanocyte stimulating hormone, and adrenocorticotropin hormone in DKO mice, which was associated with increased serum corticosterone levels and adrenal mass. DKO also increased the expression of other melanotroph-specific genes, but not corticotroph-specific genes. The dopaminergic pathway in the hypothalamus and dopaminergic receptors in melanotrophs were not affected in DKO mice. However, we observed hyperplasia of the intermediate lobe in DKO females and males, accompanied by increased POMC immunoreactivity per cell. These results indicate that PTPRN and PTPRN2 contribute to hypothalamic-pituitary-adrenal function by being involved in processes that regulate postnatal melanotroph development and Pomc expression. Therefore, although all hypothalamic and pituitary cells express Ptprn and Ptprn2 genes, their DKO affects only the function of specific cells. Further studies are needed to elucidate the mechanism of action of these genes in the affected cells [References 2 and 3].
Figure 1. Representative images of Pax7 and POMC immunostaining in the intermediate lobe (IL) and anterior lobe (AL) of control and DKO female and male mice.
The Pax7–immunopositive cells (red) were detected in the IL, while POMC immunoreactivity (green) was present in both the AL and IL of control and DKO animals. The IL of DKO mice of both sexes is larger than those from controls.
Figure 1. Representative images of Pax7 and POMC immunostaining in the intermediate lobe (IL) and anterior lobe (AL) of control and DKO female and male mice.
The Pax7–immunopositive cells (red) were detected in the IL, while POMC immunoreactivity (green) was present in both the AL and IL of control and DKO animals. The IL of DKO mice of both sexes is larger than those from controls.
Maintenance of functional pituitary gonadotrophs is dependent on phosphoinositides.
In collaboration with the group of Tamás Balla, we are also studying the functions of three phosphoinositides, PI4P, PI(4,5)P2, and PI(3,4,5)P3, in cellular signaling and exocytosis, focusing on hormone-producing pituitary cells. PI(4,5)P2, acting as a substrate for phospholipase C, plays a key role in the control of pituitary cell functions, including hormone synthesis and secretion. PI(4,5)P2 also acts as a substrate for class I PI3-kinases, leading to the generation of two intracellular messengers, PI(3,4,5)P3 and PI(3,4)P2, which act through their intracellular effectors, including Akt (a serine-threonine protein kinase). PI(4,5)P2 can also influence the release of pituitary hormones, acting as an intact lipid to regulate ion-channel gating and concomitant calcium signaling, as well as the exocytic pathway. Recent findings have also shown the expression of several PI lipid kinases, including Pi4ka, Pi4kb, Pi4k2a, Pi4k2b, Pip5k1a, Pip5k1c, and Pik3ca, as well as Pikfyve and Pip4k2c, in pituitary lactotrophs, which are responsible for prolactin secretion, a hormone that controls lactation. Using a pharmacological approach to specifically inhibit these enzymes, we showed that PI4P made in the plasma membrane by PI4KA is critical for exocytosis without affecting the calcium signals that drive secretion. Our experiments also showed that inhibition of the PI4KB enzyme, which generates PI4P in the Golgi, is dispensable for the exocytic step. These experiments revealed a key role for PI4KA–derived PI4P in the plasma membrane in calcium-secretion coupling in pituitary lactotrophs downstream of voltage-gated and PI(4,5)P2–dependent calcium signaling.
A more recent study on this topic focused on the role of PI4KA in gonadotroph function by knocking out this enzyme in cells expressing the GnRH receptor. Knockout mice were infertile, reflecting underdeveloped gonads and reproductive tracts and lack of puberty. The number and distribution of hypothalamic GnRH neurons and Gnrh1 expression in postnatal knockouts were not affected, while Kiss1/Kisspeptin expression was increased. Knockout of PI4-kinase A also did not alter embryonic establishment, neonatal development, or function of the gonadotroph population. However, during the postnatal period, there was a progressive loss of expression of gonadotroph-specific genes, including Fshb, Lhb, and Gnrhr, accompanied by low gonadotropin synthesis. The postnatal gonadotroph population also progressively declined, reaching approximately one third of that observed in controls at three months of age. In these residual gonadotrophs, GnRH–dependent calcium signaling, and calcium-dependent membrane-potential changes were lost, but intracellular administration of inositol-1,4,5-trisphosphate rescued this signaling. These results indicate a key role for PI4-kinase A in the postnatal development and maintenance of a functional gonadotroph population [Reference 4].
Distribution and calcium signaling function of somatostatin receptor subtypes in the rat pituitary
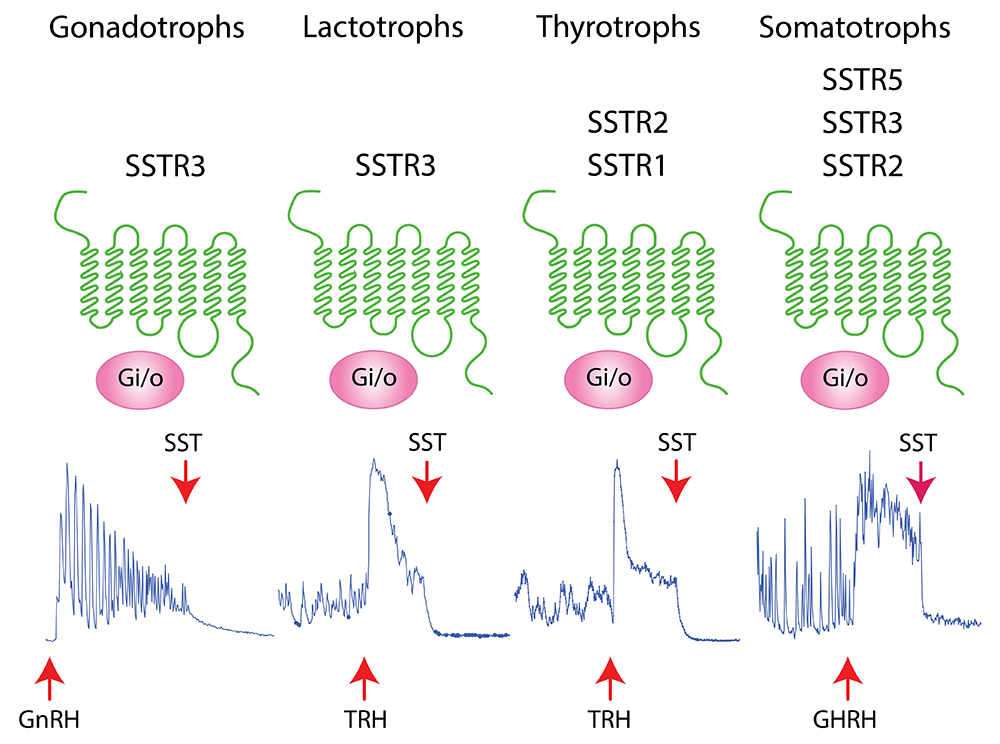
The somatostatin (SST) receptor family controls pituitary hormone secretion, but the distribution and specific roles of these receptors on the excitability and voltage-gated calcium signaling of hormone-producing pituitary cells have not been fully characterized. Our recent single-cell RNA sequencing analysis of rat pituitary cells showed cell type–specific expression of SST receptor genes in pituitary hormone–producing cells. To further advance the physiological meaning of these findings, we investigated the distribution and calcium signaling function of SST receptor functions in rat pituitary hormone–producing cells. The rat pituitary gland expressed Sstr1, Sstr2, Sstr3, and Sstr5 receptor genes in a cell type–specific manner: Sstr1 and Sstr2 in thyrotrophs, Sstr3 in gonadotrophs and lactotrophs, Sstr2, Sstr3, and Sstr5 in somatotrophs, and none in corticotrophs or melanotrophs (Figure 2). Most gonadotrophs and thyrotrophs spontaneously fired high-amplitude single action potentials, which were silenced by SST without affecting intracellular calcium concentrations. In contrast, lactotrophs and somatotrophs spontaneously fired low-amplitude plateau-bursting action potentials in conjunction with calcium transients, both of which were silenced by SST. Moreover, SST inhibited protein-coupled receptor-induced voltage-gated calcium signaling and hormone secretion in all cell types expressing SST receptors, but the inhibition was more pronounced in somatotrophs. The pattern of inhibition of electrical activity and calcium signaling was consistent with both direct and indirect inhibition of voltage-gated calcium channels, the latter being driven by cell type–specific hyperpolarization. These finding indicate that the action of SST in somatotrophs is enhanced by the expression of several types of SST receptors and their slow desensitization, that SST may play a role in the electrical resynchronization of gonadotroph, thyrotrophs, and lactotrophs, and that lack of SST receptors in corticotrophs and melanotrophs keeps them excitable and ready to respond to stress [Reference 5].
Figure 2. Expression and calcium signaling function of somatostatin receptors (SSTR) in hormone-producing pituitary cells.
The upper panel illustrates the cell types and their respective SSTRs. The lower panel illustrates typical patterns of calcium signaling induced by gonadotropin-releasing hormone (GnRH), thyrotropin-releasing hormone (TRH), and growth hormone–releasing hormone (GHRH).
Figure 2. Expression and calcium signaling function of somatostatin receptors (SSTR) in hormone-producing pituitary cells.
The upper panel illustrates the cell types and their respective SSTRs. The lower panel illustrates typical patterns of calcium signaling induced by gonadotropin-releasing hormone (GnRH), thyrotropin-releasing hormone (TRH), and growth hormone–releasing hormone (GHRH).
Publications
- Common and female-specific roles of protein tyrosine phosphatase receptors N and N2 in mice reproduction. Sci Rep 2013 13 (1):355
- Protein tyrosine phosphatase receptors N and N2 control pituitary melanotroph development and POMC expression. Endocrinology 2024 165(8):bqae076
- What is known and unknown about the role of neuroendocrine genes Ptprn and Ptprn2. Front Endocrinol 2024 submitted
- Postnatal development and maintenance of functional pituitary gonadotrophs is dependent on PI4-kinase A. Endocrinology 2023 164(12):bqad168
- Distribution and calcium signaling function of somatostatin receptor subtypes in rat pituitary. Cell Calcium 2024 124:102967
Collaborators
- Tamás Balla, MD, PhD, Section on Molecular Signal Transduction, NICHD, Bethesda, MD
- Patrick A. Fletcher, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Hana Zemkova, PhD, Institute of Physiology of the Czech Academy of Sciences, Prague, Czech Republic
Contact
For more information, email stojilks@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/stojilkovic.