You are here: Home > Section on Medical Neuroendocrinology
Diagnosis, Localization, Pathophysiology, and Molecular Biology of Pheochromocytoma and Paraganglioma

- Karel Pacak, MD, PhD, DSc, Head, Section on Medical Neuroendocrinology
- Karen T. Adams, MSc, CRNP, Research Nurse
- Stephanie Fliedner, MS, Pre-Visiting Fellow
- Thanh-Truc Huynh, MS, Biologist
- Kathryn King, BS, Postbaccalaureate Fellow
- Lucia Martiniova, MS, Predoctoral Fellow
- Maria Pavlatou, MD, PhD, Postdoctoral Fellow
- Stephen Uyeno, BS, Volunteer
We conduct patient-oriented research into the etiology, pathophysiology, genetics, diagnosis, and treatment of pheochromocytoma (PHEO) and paraganglioma (PGL). Projects include not only translational research—applying basic science knowledge to clinical diagnosis, pathophysiology, and treatment—but also “reverse translation research” by which clinical findings lead to new concepts for pursuit by basic researchers in the laboratory. Our goals are to (1) establish new and improved methods and strategies for diagnosis and localization of PHEO and PGL; (2) explain the molecular basis for varying clinical presentations of PHEO and PGL and establish the pathways of tumorigenesis; (3) search for new molecular and genetic markers for diagnosis and treatment of malignant PHEO and PGL; and (4) facilitate new and improved collaborations and interdisciplinary studies. To achieve these goals, we base our strategy on multidisciplinary collaborations with investigators from several NIH Institutes and outside medical centers. We link a patient-oriented component with two bench-level components. The patient-oriented component (medical neuroendocrinology) is the driving force for our hypotheses and discoveries. The two bench-level components (tumor pathogenesis and chemistry; biomarkers) emphasize, first, technologies of basic research tailored for pathway and target discovery and, second, the development of discoveries into clinical applications.
Hereditary PHEOs and PGLs
Advances in genetics and the recognition of the high prevalence of PHEO/PGL in certain familial syndromes dictate mandatory tumor screening in patients with those syndromes, irrespective of the presence of classical clinical signs and symptoms. Accumulating data also indicate that many more PHEOs/PGLs result from germline mutations than previously recognized, raising the importance of considering an underlying hereditary condition even in those patients without an obvious family history. To date, mutations in five main genes have been identified as responsible for familial PHEOs/PGLs: (1) the von Hippel-Lindau (VHL) gene in VHL syndrome; (2) the RET gene in multiple endocrine neoplasia type 2 (MEN 2); (3) the neurofibromatosis type 1 (NF-1) gene associated with von Recklinghausen’s disease; and (4) mutations in genes encoding mainly the B and D subunits of mitochondrial succinate dehydrogenase (SDHB and SDHD) associated with familial PHEOs/PGLs.
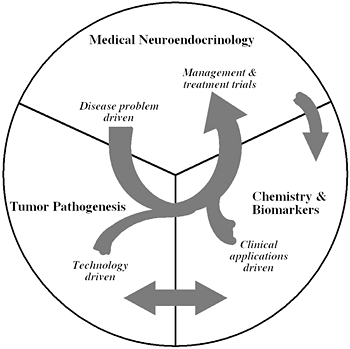
Figure 1
The unique structure of our Section tightly links together a patient-oriented component with two bench-level components. One of the latter two components emphasizes new technologies of basic research tailored for pathway and target discovery; the other focuses on further development of those and other discoveries into clinical applications.
Extra-adrenal PHEOs, otherwise known as PGLs, account for about 20% of catecholamine-producing tumors. Catecholamine excess and mutations in the genes encoding succinate dehydrogenase subunits (SDHx) are frequently found in patients with PGLs. Only 2% of PGLs are found in the mediastinum, and little is known about genetic alterations in patients with mediastinal PGLs, catecholamine production by these tumors, or their clinical behavior. We hypothesized that most mediastinal PGLs are associated with SDHx mutations, norepinephrine and/or dopamine excess, and aggressive behavior. The objective of this study was to characterize genetic, biochemical, and clinical data on a series of 10 patients with mediastinal PGLs. All 10 primary mediastinal PGL patients had germline SDHx mutations, 6 in SDHB and 4 in SDHD genes. Chest or back pain was the most common presenting symptom (5 patients), and catecholamines and/or their metabolites were elevated in 7 patients. Additional tumors included head and neck PGLs in 4 patients, PHEO in 1 patient, and bladder PGL in another. Metastatic disease was documented in 6 patients (60%), and a concurrent abdominal mass was found in one patient. We concluded that mediastinal PGLs were strongly associated with SDHB and SDHD gene mutations, noradrenergic phenotype, and aggressive behavior. The present data suggest that all patients with mediastinal PGLs should be screened for SDHx gene mutations, regardless of age. We also found that SDHx mutations are prevalent in pediatric and adult PGLs of the organ of Zuckerkandl. In addition, PGLs of the organ of Zuckerkandl are strongly associated with noradrenergic phenotype and aggressive behavior. The identification of SDHx mutations in patients with organ of Zuckerkandl PGLs has important implications for patient care and screening of family members. Such patients should be screened for SDHx mutations; in addition asymptomatic carriers of an SDHx mutation among the relatives of affected patients may benefit from tumor screening for early PGL detection. In another study of about 21 children entered into the protocol over more than 10 years, we found that familial PHEO/PGL is presented in more than 50% of these patients. We now recommend that screening of these patients start at age 5. Recent data suggest that patients with SDHB-related metastatic PHEO/PGL can have better initial responses to chemotherapy (so called CVD regimen) than other types of PHEO/PGL.
Imaging of various PHEOs and PGLs
We compared functional imaging modalities including positron emission tomography (PET) with 6-[18F]-fluorodopamine ([18F]-DA) against [123I]-metaiodobenzylguanidine ([123I]-MIBG) and somatostatin receptor scintigraphy (SRS) with [111In]-pentetreotide (Octreoscan) in non-metastatic and metastatic PHEO/PGL. We studied 25 men and 28 women (mean age±SD: 44.2±14.2 years) with biochemically-proven non-metastatic (n: 17) or metastatic (n: 36) PHEO/PGL. Evaluation included anatomical imaging with computed tomography (CT) and/or magnetic resonance imaging (MRI) and functional imaging that included at least two nuclear medicine modalities: [18F]-DA-PET, [123I]-MIBG scintigraphy, or SRS. Sensitivity of functional imaging vs. anatomical imaging was assessed on a per-patient and on a per-region basis. For this available cohort, on a per patient basis, overall sensitivity (combined for non-metastatic and metastatic PHEO) was 90.2% for [18F]-DA-PET, 76.0% for [123I]-MIBG scintigraphy, and 22.0% for SRS. On a per-region basis, overall sensitivity was 75.4% for [18F]-DA PET, 63.4% for [123I]-MIBG scintigraphy, and 64.0% for SRS. Given that PET is more sensitive than [123I]-MIBG scintigraphy or SRS, [18F]-DA PET should be used, if available, in the evaluation of PHEO/PGL. If [18F]-DA PET is not available, [123I]-MIBG scintigraphy (for non-metastatic/adrenal PHEO) and SRS (for metastatic PHEO) should be the first choice of imaging methods.
In another study, we found that among 140 patients with definitive biochemical proof of or exclusions of the presence of PHEO/PGL (91 positive, 49 negative), [123I]-MIBG scintigraphy had sensitivity and specificity of 82%. For patients evaluated for suspected disease, sensitivity and specificity were 88% and 84%, respectively. For the subpopulations of adrenal PHEO and extra-adrenal PGL, sensitivities were 88% and 67% respectively. Addition of SPECT increased reader confidence but had minimal effect on sensitivity and specificity. This prospective study demonstrated sensitivity of 82-88% and specificity of 82-84% for [123I]-MIBG scintigraphy used in the diagnostic assessment of primary or metastatic PHEO or PGL.
We also found that intravenous low-osmolar contrast-enhanced CT can safely be used in PHEO/PGL patients without alpha or beta-adrenergic blockade.
Other clinical findings
Signs and symptoms associated with PHEO/PGL are predominantly caused by catecholamine excess, but tend to be highly variable and non-specific. In this study, we evaluated 23 male and 35 female PHEO/PGL patients for signs and symptoms of PHEO/PGL with special regard to gender-related differences in presentation. Total symptom score comparison between genders showed significant differences (12.0 vs. 7.8, P-value 0.0001). Female patients reported significantly more headache (80% vs. 52%), dizziness (83% vs. 39%), anxiety (85% vs. 50%), tremor (64% vs. 33%), weight change (88% vs. 43%), numbness (57% vs. 24%), and changes in energy level (89% vs. 64%). Females and males displayed comparable biochemical phenotypes (60% and 65% noradrenergic phenotype, respectively). Use of alpha and/or beta blockade did not differ significantly between males and females. Subgroup analyses and multiple regression analysis revealed gender differences, irrespective of benign or malignant disease, use of adrenoceptor-blockade, age, or biochemical phenotype. We concluded that female patients had significantly more self-reported PHEO/PGL signs and symptoms than male patients, irrespective of biochemical phenotype and tumor presentation; this may be related to distinct catecholamine receptor sensitivity. Clinicians should be aware of these complaints in female PHEO/PGL patients and offer adequate treatment if indicated.
To establish if classification of patients with SDHB-related metastatic PHEO/PGL, based on a characteristic urinary peptide pattern, is possible, urine samples from patients with SDHB-derived metastatic PHEO/PGL (SDHB-met, n=11) were compared with samples from SDHB-derived non-metastatic PHEO/PGL (SDHB-nm, n=7) and healthy SDHB mutation carriers (SDHB-hlty, n=12). Differential urine peptide analysis was performed by capillary electrophoreses (CE) coupled with electrospray ionization time-of-flight mass spectrometry (TOF-MS). After normalization of CE-migration time and signal intensity, peptide peaks of various samples were matched to abundant "housekeeping" polypeptides; urinary peptide patterns were compiled. Peptides of interest were identified by matching of retention time and molecular weight to a urine peptide database as well as by various MS/MS approaches; this study is ongoing. Two peptides, always among the best 5 peptides to distinguish SDHB-met and SDHB-hlty, were identified as fragments of collagen alpha-1 (III) (COL3A) chain. A closer look at these fragments revealed that absence of peptide 76415 correlates particularly well with metastatic disease. Only 1 of the 12 patients with metastatic disease showed this peptide; however, the peptide was found in 16 of the 18 patients without metastases (SDHB-nm and SDHB-hlty). Development of EIA (enzyme immunoassay) testing for the presence or absence of COL3A fragment 76415 may allow for new screening and follow-up measures in metastatic disease.
An animal model of PHEO and cell culture studies
Treatment of metastatic PHEO/PGL requires proper diagnostic imaging. An animal model of metastatic PHEO/PGL was previously developed; the aim of this study was to evaluate the feasibility of small animal PET, micro-computed tomography (microCT), and MRI to detect metastatic lesions in this model. Furthermore, we compared the efficiency of two major radiopharmaceuticals [18F]-DA and [18F]-L-6-fluoro-3,4-dihydroxyphenylalanine ([18F]-DOPA) for detection of subcutaneous PHEO with small animal PET. We studied 18 nude mice: 5 x 106 injected subcutaneously (n = 4) and 1 x 106 injected intravenously (n = 9) with mouse pheochromocytoma cells (MPCs 4/30PRR) cells and 5 controls. Metastatic lesions were first localized with T2-weighted, respiratory-triggered MRI, and microCT. Each animal then underwent [18F]-DA and [18F]-DOPA PET scans. Dynamic and whole body PET images were collected to assess the optimal scanning time after SUVmax was calculated for tumors and highest tumor-to-liver ratio (TLR) was evaluated. Histology was performed to verify PHEO/PGL origin. The optimal time to scan for [18F]-DOPA animal PET was found to be 60-70 minutes after injection of the tracer—when the TLR value reached its peak. The TLR for liver lesions with [18F]-DA was 1.8-2.8 depending on the tumor size. TLR value for liver lesions with [18F]-DOPA was 2.2-2.7. [18F]-DA reveals very low accumulation in both smaller and larger subcutaneous tumors (TLR=0.22-0.27), almost at the level of background within muscle. In comparison, the uptake of [18F]-DOPA within the same tumors was much higher (TLR=1.56-2.24). We concluded that [18F]-DOPA animal PET is an excellent approach to study subcutaneous PHEO/PGL metastatic lesions—an approach that can be used in the development of new therapeutic strategies.
Publications
- Timmers HJ, Pacak K, Bertherat J, Lenders JW, Eisenhofer G, Stratakis CA, Nicoli-Sire P, Huy PT, Burnichon N, Gimenez-Raqueplo AP. Mutation associated with succinate dehydrogenase d-related malignant pheochromocytoma. Clin Endocrinol 2008 64:561-566.
- Baid S, Lai EW, Wesley RA, Ling A, Adams KT, Kozupa A, Pacak K. Contrast enhanced computed tomography does not induce catecholamine release in patients with pheochromocytoma. Ann Int Med 2009 150:27-32.
- Ghayee HK, Havekes B, Corssmit EPM, Eisenhofer G, Hammes SR, Ahmad Z, Tessnow A, Lazurova I, Adams KT, Fojo AT, Pacak K, Auchus RJ. Mediastinal paragangliomas: association with mutations in the succinate dehydrogenase genes and aggressive behavior. Endocr Relat Cancer 2009 16:291-299.
- Timmers H, Gimenez-Roqueplo AP, Manelli M, Pacak K. Clinical aspects of SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer 2009 16:391-400.
- Lai EW, Joshi BH, Martiniova L, Dogra R, Fujisawa T, Leland P, Krijger RR, Lubensky IA, Elkahloun AG, Morris JC, Puri RK, Pacak K. Overexpression of interleukin-13 receptor-alpha2 in neuroendocrine malignant pheochromocytoma: a novel target for receptor directed anti-cancer therapy. J Clin Endocrinol Metab 2009 94:2952-2957.
Collaborators
- Mones Abu-Asab, PhD, Pathology Department, Clinical Center, NIH, Bethesda, MD
- Chris Albanese, PhD, Georgetown University, Washington, DC
- Jorge A. Carrasquillo, MD, Memorial Sloan-Kettering Cancer Center, New York, NY
- Clara C. Chen, MD, Nuclear Medicine Department, Clinical Center, NIH, Bethesda, MD
- Graeme Eisenhofer, PhD, Universität Dresden, Dresden, Germany
- Abdel G. Elkahloun, PhD, Genome Technology Branch, NHGRI, NIH, Bethesda, MD
- Tito Fojo, MD, PhD, Medical Oncology Branch, NCI, Bethesda, MD
- G&B Solutions Inc., McLean, VA
- Jeff Green, MD, PhD, Laboratory of Cancer Biology and Genetics, NCI, Bethesda, MD
- Ioannis Ilias, MD, University of Patras, Patras, Greece
- Electron Kebebew, MD, Surgery Branch, NCI, Bethesda, MD
- Jacques Lenders, MD, St. Radboud University, Nijmegen, The Netherlands
- W. Marston Linehan, MD, Urologic Oncology Branch, NCI, Bethesda, MD
- Alexander Ling, MD, Radiology Department, Clinical Center, NIH, Bethesda, MD
- Irina A. Lubensky, MD, Cancer Diagnosis Program, NCI, Bethesda, MD
- Maria J. Merino, MD, Pathology Department, NCI, Bethesda, MD
- John C. Morris, MD, PhD, Metabolism Branch, NCI, Bethesda, MD
- Peter J. Munson, PhD, Center for Information Technology, NIH, Bethesda, MD
- Alan Pang, MD, PhD, Program in Reproductive and Adult Endocrinology, NICHD, Bethesda, MD
- Forbes D Porter, MD, PhD, Program in Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- Raj K. Puri, MD, PhD, Center for Biologics Evaluation and Research, FDA, Rockville, MD
- Margarita Raygada, PhD, Program in Reproductive and Adult Endocrinology, NICHD, Bethesda, MD
- James C. Reynolds, MD, Nuclear Medicine Department, Clinical Center, NIH, Bethesda, MD
- Constantine A. Stratakis, MD, D(med)Sci, Program in Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- David Thomasson, PhD, Radiology Department, Clinical Center, NIH, Bethesda, MD
- Henri Timmers, MD, PhD, St. Radboud University, Nijmegen, The Netherlands
- Arthur S. Tischler, MD, PhD, New England Medical Center, Boston, MA
- Robert A. Wesley, PhD, Clinical Center, NIH, Bethesda, MD
- Jiri Widimsky, MD, PhD, Charles University, Prague, Czech Republic
- Zhengping Zhuang, MD, PhD, Surgical Neurology Branch, NINDS, NIH, Bethesda, MD
Contact
For more information, email karel@mail.nih.gov.



