You are here: Home > Unit on Microbial Pathogenesis
Modulation of Human Macrophages by the Intracellular Pathogen Legionella pneumophila

- Matthias Machner, PhD, Head, Unit on Microbial Pathogenesis
- Maria Ramona Aldea, PhD, Postdoctoral Fellow
- Yang Chen, BA, Graduate Student
- Andrew Gaspar, PhD, Postdoctoral Fellow
- Antonia Thomas, BA, Summer Internship Student
We study the molecular details underlying survival and replication of Legionella pneumophila within human cells. Bacterial pathogens have developed a variety of strategies to exploit the human host and cause disease. Many Gram-negative bacteria use type IV secretion systems (T4SSs) to deliver bacterial toxins into host cells, where they modulate signaling events to create an environment supportive for bacterial survival and growth. Legionella pneumophila is an opportunistic bacterial pathogen that causes, in humans, a life-threatening pneumonia called Legionnaires' disease. Upon phagocytosis by alveolar macrophages, the organism employes a Dot/Icm T4SS to deliver a large number of bacterial effector proteins into the host cell cytosol. These effectors allow L. pneumophila to intercept proteins and small transport vesicles of the infected cell, thereby transforming the Legionella-containing vacuole (LCV) into a protective compartment resembling host-cell endoplasmic reticulum (ER). Our main goal is to determine the molecular function of these translocated effectors and to identify the host cell pathways modulated by these proteins.
Bacterial effector proteins in L. pneumophila pathogenesis
We investigate the molecular mechanisms by which the intracellular pathogen Legionella pneumophila can exploit human cells and cause disease. The organism enters the human lung through inhalation, where it infects alveolar macrophages (Figure 1). Upon phagocytosis, L. pneumophila avoids lysosomal fusion and recruits proteins and transport vesicles from the host-cell cytosol to transform its phagosome into a specialized vacuole that resembles host-cell rough endoplasmic reticulum (ER). Within this protective ER-like compartment L. pneumophila can replicate to high numbers, eventually lyze the host cell, and infect neighboring macrophages. Replication vacuole formation and intracellular survival of L. pneumophila depend on a functional type IV secretion system named Dot/Icm. This multiprotein complex delivers a large number of L. pneumophila effector proteins into the host cell cytosol. Most of these effectors are unique to the genus Legionella, display no sequence homology to other proteins, and their function is unknown. They are, however, of critical importance for L. pneumophila virulence, given that mutants with a defective Dot/Icm translocation system are unable to survive within host cells and cause disease. Our laboratory aims to elucidate the molecular role of L. pneumophila effector proteins and how they contribute to hijacking proteins and membrane material from the infected host cell.
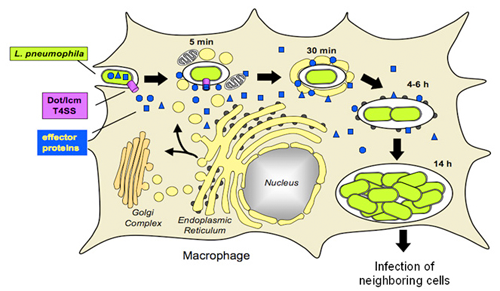
Figure 1. Legionella pneumophila intracellular replication cycle
L. pneumophila uses the Dot/Icm T4SS (white) to deliver a multitude of effector proteins (black) into the cytosol of an infected macrophage. The pathogen recruits host cell transport vesicles to establish a replication vacuole.
Rab1 modulation by the L. pneumophila effector protein SidM
One of the Dot/Icm substrates discovered in our laboratory is SidM. Shortly after bacterial uptake, SidM is delivered by the Dot/Icm system into the host cell, where it localizes to the surface of the LCV. Using purified SidM as bait in pulldown studies, we found that SidM specifically interacts with host cell Rab1. Rab1 is a small GTPase involved in regulating secretory vesicle trafficking from the ER to the Golgi complex. Rab proteins function as molecular switches that cycle between an active GTP conformation and an inactive GDP-bound form. Activation of Rab proteins is catalyzed by GDP/GTP exchange factors (GEFs) whereas GTPase-activating proteins (GAPs) inactivate Rabs by triggering their intrinsic GTP hydrolysis activity. Our in vitro binding studies revealed that L. pneumophila SidM preferentially interacts with the inactive form of Rab1 but not with GTP-Rab1. We also found that SidM catalyzes exchange of GDP against GTP in Rab1, consistent with SidM being a Rab1-GEF.
Within eukaryotic cells, GDP-Rab proteins are complexed by a cytosolic chaperone called GDP dissociation inhibitor (GDI). GDI protects the hydrophobic geranylgeranyl anchor covalently attached to the C-terminus of Rab proteins from the aqueous environment of the cytosol and prevents unrestricted activation of Rabs. Release of Rab proteins from GDI prior to activation by GEFs is believed to require a protein with GDI displacement factor (GDF) activity. Using purified proteins in studies in vitro, we found that SidM possessed GDF activity and triggered release of prenylated Rab1 from its chaperone GDI. Both GEF and GDF activity required the central region of SidM that mediated Rab1 binding.
Rab1 has previously been found to colocalize with LCVs as early as 10 minutes after bacterial internalization by phagocytes. This process is dependent on a functional Dot/Icm T4SS, suggesting that translocated effector protein(s) of L. pneumophila are involved in this recruitment process. Our studies have now revealed that vacuoles containing L. pneumophila sidM mutants do not display any detectable colocalization with Rab1, indicating that Rab1 recruitment requires SidM on the surface of LCVs. These findings suggest that it is the unusual ability of SidM to combine GDF and GEF activity that allows L. pneumophila to efficiently exploit the cytosolic pool of host cell Rab1 during infection (Figure 2).
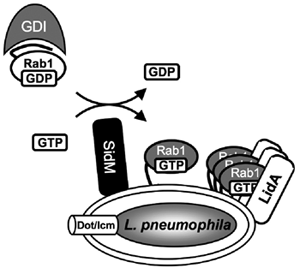
Figure 2. Molecular mechanism of Rab1 recruitment.
Legionella pneumophila effector proteins exploit host cell Rab1. SidM recruits Rab1 to the Legionella-containing vacuole and activates it through GDP/GTP exchange. Activated GTP-Rab1 is released by SidM and accumulated on the vacuole by LidA.
Targeting of Rab GTPases by the L. pneumophila effector protein LidA
Our search for other Rab1-interacting partners revealed a second protein from L. pneumophila named LidA. Like SidM, LidA is a translocated substrate of the Dot/Icm T4SS that localizes to the surface of the LCV. Immunofluorescence microscopy studies revealed that vacuoles containing L. pneumophila lidA mutants colocalize with Rab1, indicating that LidA is not absolutely required for Rab1 recruitment. Enzyme-linked immunosorbent assay (ELISA) studies showed that purified recombinant LidA interacted with GDP- as well as GTP-bound Rab1. Unlike SidM, LidA has neither GEF nor GDF activity, explaining why this effector is not sufficient to mediate recruitment and activation of Rab1 in the absence of SidM. Mutants lacking LidA, however, showed a kinetic defect in Rab1 recruitment, suggesting that LidA is important for efficient Rab1 accumulation at the LCV (Figure 2). In addition to Rab1, LidA also interacted with the host GTPases Rab6 and Rab8 involved in the regulation of Golgi-directed transport vesicles. Thus, L. pneumophila appears to possess effector proteins to target multiple host cell pathways simultaneously.
Publications
- Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell 2006 11:47-56.
- Machner MP, Isberg RR. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science 2007 318:974-977.
Collaborators
- Wolf-Dieter Schubert, PhD, Helmholtz Center for Infection Research, Braunschweig, Germany
- Roger Goody, PhD, Max Planck Institute for Molecular Physiology, Dortmund, Germany
Contact
For more information, email machnerm@mail.nih.gov or visit cbmp.nichd.nih.gov/ump/research.html



