You are here: Home > Section on Cellular Signaling
Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Melanija Tomić, PhD, Staff Scientist
- Ivana Bjelobaba, PhD, Visiting Fellow
- Marija M. Janjic, PhD, Visiting Fellow
- Ellias Leiva-Salcedo, PhD, Visiting Fellow
- Milos B. Rokic, PhD, Visiting Fellow
- Jovana Tavcar, MD, Special Volunteer
Using multidisciplinary and collaborative approaches, we investigate receptors and ligand-gated channels, their activation by hormones and neurotransmitters, and their roles in intracellular signaling, gene expression, and hormone secretion in neuroendocrine cells. The work includes characterization of native and recombinant receptors and channels that were cloned from these cells. Currently, we are studying the calcium-mobilizing receptor-coupled gene network in non-transformed cells using RNA sequencing and qRT-PCR analysis of primary pituitary cells from developing and postpubertal mice and rats. We are also determining how the structural features of pituitary channels relate to the channels' functions and how plasma membrane receptors and the intracellular signaling milieu affect channel activity.
GnRH and TRH induction of pituitary gonadotroph genes
Click image to enlarge.
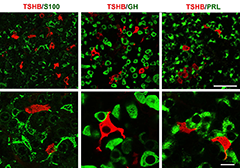
Figure 1. Double immunofluorescent labeling for TSHB (red fluorescence) and S100, GH or PRL (green fluorescence) in anterior pituitary sections of postpubertal female rats
Left: Organization of S100–positive folliculostellate cells and their relationship with thyrotrophs. Middle: Dominance of GH–positive cells in anterior pituitary tissue and their interactions with thyrotrophs. Right: Interconnections between PRL–positive cells and their physical closeness to thyrotrophs.
Hypothalamic gonadotropin-releasing hormone (GnRH) is the primary regulator of reproduction in vertebrates, acting via the G protein–coupled GnRH receptor (GnRHR) expressed in pituitary gonadotrophs to control synthesis and release of gonadotropins. Recently, we sought to increase our understanding of the GnRHR–coupled gene network in non-transformed cells, using RNA sequencing of primary pituitary cells from postpubertal female rats treated with GnRH. The study revealed 83 candidate regulated genes, including many coding for secreted proteins. Previously, we had characterized GnRH induction of the gene encoding dentin matrix protein-1 (Dmp1), one of five members of the small integrin-binding ligand N-linked glycoprotein gene family (1). The focus in ongoing investigations is on GnRH stimulation of gonadotroph-specific genes: luteinizing hormone beta chain (Lhb), follicle-stimulating hormone beta chain (Fshb), GnRH receptor (Gnrhr), secreted phosphoprotein 1 (Spp1), also known as osteopontin, and glycoprotein alpha chain (Cga), also expressed in thyrotrophs. GnRH was applied for five minutes once or twice per hour or continuously for six hours, and the abundance of these transcripts was measured by qRT-PCR. As in RNA sequencing experiments, we observed about 50% increases in Cga expression when GnRH was applied either in two five-minute pulses per hour or continuously, but not in cells pulsed once per hour. In further agreement with the RNA–sequencing analysis, no increase in Lhb expression was observed. Frequency coding was critical for GnRH–induced Fshb expression, whereas both periodic and continuous application of GnRH triggered Gnrhr expression. The experiments, still in progress, indicate that a six-hour exposure to GnRH is a sufficient to increase the abundance of three out of four signature transcript in perifused pituitary cells from postpubertal female rats.
We also studied the in vivo and in vitro expression pattern of three genes that are operative in the thyrotroph subpopulation of anterior pituitary cells: Cga, thyroid-stimulating hormone beta chain (Tshb), and thyrotropin-releasing hormone receptor (Trhr). In vivo, the expression of Cga and Tshb was robust, whereas the expression of Trhr was low. In cultured pituitary cells, there was a progressive decline in the expression of Cga, Tshb, and Trhr. The expression of Tshb could not be reversed by pulsatile or continuous thyroid releasing hormone (TRH) application in variable concentrations and treatment duration, or by the removal of thyroid and steroid hormones from the sera. In parallel, the expression of CGA and TSHB proteins declined progressively in pituitary cells from both sexes. The lack of the effect of TRH on Tshb expression was not related to the age of pituitary cultures or the presence of functional TRH receptors. In cultured pituitary fragments, there was also a rapid decline in expression of these genes but TRH was able to induce transient Tshb expression. In vivo, thyrotrophs were often in close proximity to each other and to somatotroph and folliculo-stellate cell networks, and especially to the lactotroph cell network (Figure 1); the organization pattern was lost in vitro. These observations suggest that the lack of influence of anterior pituitary architecture and/or intrapituitary factors probably accounts for the loss of basal and TRH–stimulated Tshb expression in dispersed pituitary cells (2).
Differential effect of paliperidone and aripiprazole on the strength of calcium-secretion coupling
Hyperprolactinemia is a common adverse in vivo effect of antipsychotic medications that are used in the treatment of patients with schizophrenia. We compared the effects of two atypical antipsychotics, paliperidone and aripiprazole, on cAMP/calcium signaling and prolactin (PRL) release in female rat pituitary lactotrophs in vitro. Dopamine inhibited spontaneous cAMP/calcium signaling and PRL release. In the presence of dopamine, paliperidone rescued cAMP/calcium signaling and PRL release in a concentration-dependent manner, whereas aripiprazole was only partially effective. In the absence of dopamine, paliperidone stimulated cAMP/calcium signaling and PRL release, whereas aripiprazole inhibited signaling and secretion more potently but less effectively than dopamine. Forskolin-stimulated cAMP production was facilitated by paliperidone and inhibited by aripiprazole, although the latter was not as effective as dopamine. None of the compounds affected PRL transcript activity, intracellular PRL accumulation, or growth hormone (GH) secretion. The experiments, still in progress, indicate that paliperidone has dual hyperprolactinemic actions in lactotrophs: (i) by preserving the coupling of spontaneous electrical activity and PRL secretion in the presence of dopamine; and (ii) by inhibiting intrinsic dopamine receptor activity in the absence of dopamine, leading to enhanced calcium signaling and secretion. In contrast, aripiprazole acts on PRL secretion by attenuating, but not abolishing, calcium-secretion coupling. Thus, with regard to changes in lactotroph function by these antipsychotics, aripiprazole has obvious advantages over paliperidone.
Gating properties of pituitary purinergic receptor channels
The other main focus in our investigations is on structural and functional characterization of the two ATP–gated purinergic (P2X) receptor subtypes P2X7 and P2X4, natively expressed in pituitary cells (3). The P2X7 receptor operates as a cytolytic and apoptotic receptor but also controls sustained cellular responses, including cell growth and proliferation. However, it has not been clarified how the same receptor mediates such opposing effects. In previous work, we showed that the same receptor is capable of exhibiting sensitization and pore dilation (leading to the formation of biphasic currents and cell death) and/or desensitization (leading to a decline in current amplitude and cell-life signaling) during sustained application of orthosteric agonists, depending on their concentrations. This was accomplished in part by developing a 12-state Markov model consisting of naive, desensitized, and sensitized/dilated states that reproduced whole-cell current recordings generated experimentally. In the model, we assumed that the occupancy of one or two ATP–binding sites of naive receptors favored transition from open to desensitized states, whereas the occupancy of the third binding site favored receptor sensitization. More recently, we extend the modeling study by examining how the model cell behaves under various ionic conditions in the medium, including those that contain the large organic cation N-methyl-ᴅ-glucamine or divalent cations such as calcium. We illustrated how the two main gating patterns behave under these ionic conditions and determined why the shift in reversal potential and the dilation of the channels are accompanied paradoxically by a decrease in the total conductance during voltage ramp protocols. The model adds more evidence to our previous hypothesis, suggesting that dilation is masking desensitization. Our results also indicated that the allosteric sites through which divalent cations inhibit P2X7R should be located extracellularly.
Click image to enlarge.
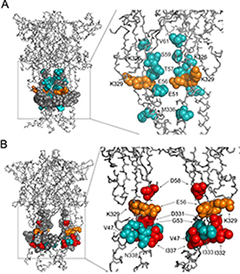
Figure 2. Topology of cadmium-affected residues in the homology model of rP2X4R
Left panel represents a wire-frame model of the rP2X4R polypeptide backbone with residues from V47–V61 and K326–N338 (spheres) in closed (A) and open (B) state. Orange spheres depict the residues in cysteine mutants that were affected by cadmium in both states. Cadmium-inhibited residue mutants are depicted by red spheres, and potentiated residue mutants are depicted by blue spheres. On the right panels, the front subunit was removed for better visibility of the vestibule interior.
We also studied the influence of allostery on channel pore dilation using the rat P2X4 receptor expressed in HEK-293T cells and gated by ATP in the presence and absence of ivermectin (IVM), an established positive allosteric regulator of this channel. In the absence of IVM, the channel activates and deactivates rapidly, does not show transition from open to dilated states, desensitizes completely with a moderate rate, and recovers only fractionally during washout. IVM treatment increases the efficacy of ATP to activate the channel and slows receptor desensitization during sustained ATP application and receptor deactivation after ATP washout. The rescue of the receptor from desensitization temporally coincides with pore dilation, and the dilated channel can be reactivated after washout of ATP. Experiments with vestibular and transmembrane domain–receptor mutants further established that IVM has distinct effects on opening and dilation of the channel pore, the first accounting for increased peak current amplitude and the latter correlating with changes in the agonist potency and kinetics of receptor deactivation. The corresponding kinetic model indicates that the IVM–dependent transition from an open to a dilated state is coupled to receptor sensitization, which rescues the receptor from desensitization and subsequent internalization. Allosterically induced sensitization of the P2X4 receptor thus provides sustained signaling during prolonged and repetitive ATP stimulation (4).
P2X receptors consist of three subunits that are mutually intertwined and form an upper, central, and extracellular vestibule with three lateral portals and the channel pore. We used cysteine- and alanine-scanning mutagenesis of the rat P2X4 receptor V47–V61 and K326–N338 sequences to study structural and functional properties of the extracellular vestibule during gating. Cysteine mutants were used to test the accessibility of the residue side chains to cadmium during closed-open-desensitized transitions, while alanine mutants served as controls. The study revealed the accessibility of residues E51, T57, S59, V61, K326, and M336 to cadmium in channels undergoing a transition from a closed to an open state and the accessibility of residues V47, G53, D331, I332, I333, T335, I337, and N338 in channels undergoing a transition from an open to a desensitized state; residues E56 and K329 were accessible during both transitions. The effect of cadmium on channel gating is stimulatory in all reactive V47–V61 mutants and inhibitory in the majority of reactive K326–N338 mutants. The rat P2X4 receptor homology model suggests that residues affected by cadmium in the closed-to-open transition are located within the lumen of the extracellular vestibule and toward the central vestibule; however, the residues affected by cadmium in the open-to-desensitized state were located at the bottom of the vestibule near the pore (Figure 2). Analysis of the model assumed that there is ion access to extracellular and central vestibules through lateral ports when the channel is closed, with residues above the first transmembrane domain being predominantly responsible for ion uptake. Upon receptor activation, there is passage of ions towards the residues located on the upper region of the second transmembrane domain, followed by permeation through the gate region (5).
Publications
- Kucka M, Bjelobaba I, Clokie SJH, Klein DC, Stojilkovic SS. Female-specific induction of rat pituitary dentin matrix protein-1 by GnRH. Mol Endocrinol 2013;27:1840-1855.
- Bargi-Souza P, Kucka M, Bjelobaba I, Tomic M, Janjic MM, Nunes, MT, Stojilkovic SS. Loss of basal and TRH-stimulated Tshb expression in dispersed pituitary cells. Endocrinology 2014;1(1):242-54.
- Stojilkovic SS, Leiva-Salcedo E, Rokic MB, Coddou C. Regulation of ATP-gated P2X channels: From redox signaling to interactions with other proteins. Antioxid Redox Signal 2014;21:953-970.
- Rokic MB, Stojilkovic SS, Zemkova H. Structural and functional properties of the rat P2X4 purinoreceptor extracellular vestibule during gating. Front Cell Neurosci 2014;8:3-28.
- Zemkova H, Khadra A, Rokic MB, Tvrdonova V, Sherman A, Stojilkovic SS. Allosteric regulation of the P2X receptor channel pore dilation. Pflügers Arch 2014;E-pub ahead of print.
Collaborators
- Anmar Khadra, PhD, McGill University, Montreal, Canada
- David C. Klein, PhD, Program on Developmental Endocrinology and Genetics, NICHD, Bethesda
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Hana Zemková, RNDr, PhD, Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic
Contact
For more information, email stankos@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx.

