You are here: Home > Section on Intracellular Protein Trafficking
Protein Sorting in the Endosomal-Lysosomal System

- Juan S. Bonifacino, PhD, Head, Section on Intracellular Protein Trafficking
- Rafael Mattera, PhD, Staff Scientist
- Xiaolin Zhu, RN, Technician
- Shuhei Ishikura, PhD, Postdoctoral Fellow
- Javier G. Magadán, PhD, Postdoctoral Fellow
- Yogikala Prabhu, PhD, Postdoctoral Fellow
- Krishnakant Soni, PhD, Postdoctoral Fellow
- Kathryn E. Tifft, PhD, Postdoctoral Fellow
- Christina A. Schindler, PhD, Postdoctoral Fellow
- Ginny Farias, PhD, Visiting Fellow
- Xiaoli Guo, PhD, Visiting Fellow
- Patricia V. Burgos, PhD, Postdoctoral Fellow
- Gonzalo A. Mardones, PhD, Postdoctoral Fellow
- F. Javier Pérez-Victoria, PhD, Postdoctoral Fellow
We investigate the molecular mechanisms by which transmembrane proteins are sorted to intracellular compartments such as endosomes, lysosomes, and a group of cell type–specific organelles known as lysosome-related organelles (e.g., melanosomes and platelet dense bodies). Sorting to these compartments is mediated by recognition of signals present in the cytosolic domains of the transmembrane proteins by adaptor proteins that are components of membrane coats. Among these adaptor proteins are the heterotetrameric AP-1, AP-2, AP-3, and AP-4 complexes, the monomeric GGA1, GGA2, and GGA3 proteins (GGAs) (see image below), and the heteropentameric retromer complex. Proper sorting also requires the function of other components of the trafficking machinery that mediate vesicle tethering and fusion, such as the heterotetrameric GARP complex. Current work in our laboratory aims to elucidate the structure, regulation, and physiological roles of coat proteins and vesicle-tethering factors and to investigate pathological states involving dysfunction of these proteins (e.g., the Hermansky-Pudlak syndrome, Alzheimer's Disease, HIV pathogenesis).
Sorting of the amyloid precursor protein regulated by AP-4
The AP-4 complex is the most-recently discovered and least understood of the family of AP complexes. In recent work, we found that AP-4 interacts with the cytosolic tail of the Alzheimer's disease amyloid precursor protein (APP). Disruption of the AP-4–APP interaction shifts the distribution of APP from endosomes to the trans-Golgi network (TGN) and enhances gamma-secretase–catalyzed processing of APP to the pathogenic amyloid-beta peptide. These findings establish AP-4 as a novel regulator of APP processing and trafficking and as a potential risk factor for Alzheimer's disease.
Role of the GARP complex in transport from endosomes to the Golgi complex
The sorting of newly-synthesized acid hydrolases to lysosomes relies on mannose 6-phosphate receptors (MPRs), which bind to the hydrolases at the TGN and transport them to endosomes. The hydrolases are subsequently transported to lysosomes whereas the receptors return to the TGN to engage in additional rounds of sorting. In previous work, we showed that selection of MPRs for retrieval to the TGN is mediated by the retromer complex. We recently identified another complex named GARP, which enables tethering and fusion of MPR-containing carriers to the TGN.
We had previously shown that the human GARP complex comprises three subunits named VPS52, VPS53, and VPS54. We have now discovered that a protein previously named ANG2 is a fourth subunit of the GARP complex. Interference with any of the GARP subunits blocks the delivery of cargos such as MPRs and Shiga toxin from endosomes to the TGN. As a consequence, acid hydrolases are missorted to the extracellular space, lysosomes become dysfunctional, and cells exhibit defective lipid traffic and autophagy.
We also elucidated the structural basis for motor neuron degeneration in the VPS54-mutant "wobbler" mouse, an animal model for amyotrophic lateral sclerosis (ALS). In collaboration with Aitor Hierro, we solved the crystal structure of the C-terminal domain of VPS54, which harbors the wobbler mutation (leucine-967 to glutamine). The structure revealed that GARP is related to other multisubunit tethering complexes such as the exocyst and COG. In addition, we found that the wobbler mutation destabilizes VPS54, resulting in its enhanced degradation and decreased levels in all tissues of the mouse. Motor neuron degeneration in this mouse therefore results from decreased levels of GARP.
Characteristics of the BLOC-3 complex, which is defective in the Hermansky-Pudlak syndrome
We definitively established that the BLOC-3 complex, which is defective in some forms of Hermansky-Pudlak syndrome, a pigmentation and bleeding disorder, consists of a 1:1 assembly of two subunits: HPS1 and HPS4. In addition, we demonstrated that the complex interacts with the GTP-bound (active) form of the Rab9 GTPase, suggesting that the complex might function as a Rab9 effector in the biogenesis of melanosomes and platelet dense bodies.
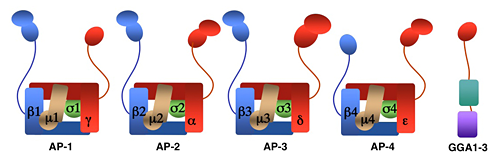
Figure 1. Structure of AP complexes and GGAs.
Mechanisms of CD4 downregulation by the Nef and Vpu proteins of HIV-1
This project aims to elucidate the mechanisms by which the Nef and Vpu proteins of human immunodeficiency virus 1 (HIV-1) downregulate CD4 in the viral host cells, T-lymphocytes, and macrophages. HIV-1 Nef is a 27-kDa myristoylated accessory protein that is produced at high levels early during infection. Nef is an important determinant of pathogenicity, as demonstrated by the finding that some long-term non-progressors (i.e., untreated infected persons who do not develop symptoms of AIDS for 10 years or longer) carry an HIV-1 strain with inactivating mutations of the Nef gene. An understanding of Nef function could thus provide new avenues for therapeutic intervention. In infected cells, Nef has many effects, the best characterized being the downregulation of CD4. Together with chemokine receptors, CD4 serves as a co-receptor for HIV-1 entry into cells. The downregulation of CD4 by Nef is thought to prevent superinfection and increase the release of infectious particles, thus explaining the higher virulence of Nef-carrying HIV-1 strains.
In previous work, we demonstrated that the downregulation of CD4 by HIV-1 Nef requires clathrin and the heterotetrameric, clathrin-associated adaptor protein-2 (AP-2) complex in host cells. In addition, we found that the requirement stems from a direct interaction of Nef with AP-2 involving a dileucine and a diacidic motif in Nef and the alpha and sigma2 subunits of AP-2. Through mutational analyses, we mapped the binding site for both Nef motifs on alpha and sigma2. Given that clathrin and AP-2 mediate endocytosis from the plasma membrane, these findings indicated that Nef downregulates CD4 by accelerating its internalization form the surface of host cells. In addition, we demonstrated that Nef has a second function: targeting internalized CD4 to the multivesicular body (MVB) pathway for eventual degradation in lysosomes. We showed that this targeting depends on ESCRT complexes but, surprisingly, not on ubiquitination of lysine residues in either CD4 or Nef.
At later stages of infection, the HIV-1 genome directs expression of a second protein, Vpu, which downregulates CD4 from the endoplasmic reticulum (ER). Vpu is a type I integral membrane protein that inserts into the membrane of the ER and targets newly synthesized CD4 for degradation by cytosolic proteasomes. This targeting was thought to be distinct from the canonical ER-associated degradation (ERAD) pathway. In collaboration with Klaus Strebel and Yihong Ye, we recently demonstrated, however, that CD4 downregulation by Vpu involves at least part of the ERAD machinery. In particular, we found that the p97-Npl4-Ufd1 ERAD dislocase is required for Vpu-induced CD4 targeting for proteasomal degradation. We also found that targeting involves CD4 ubiquitination that is dependent on not only lysine but also on serine and threonine residues. Finally, we demonstrated that Vpu also induces CD4 retention in the ER largely by virtue of transmembrane-domain interactions. The multiple levels at which Vpu engages these cellular quality control mechanisms underscore the importance of ensuring profound suppression of CD4 to the life cycle of HIV-1.
Finally, we explored how common non-lysine–dependent ubiquitination is as a signal for ERAD. To this end, we examined the degradation of the alpha subunit of the T-cell antigen receptor complex (TCR-alpha), a prototypical ERAD substrate. TCR-alpha is a type I integral membrane protein that becomes ubiquitinated and targeted to ERAD when it fails to assemble into the TCR complex. Remarkably, TCR-alpha has a cytosolic tail of only five amino acid residues (i.e., RLWSS), none of which is the canonical ubiquitin-acceptor lysine. We found that substitution of two conserved serine residues in the cytosolic tail of TCR-alpha by alanine decreased ubiquitination, whereas placement of additional serine residues enhanced it. Moreover, replacement of the cytosolic serine residues by other ubiquitinatable residues (i.e., cysteine, threonine, or lysine) allowed ubiquitination to take place. Serine-dependent ubiquitination perfectly correlated with targeting of TCR-alpha for ERAD. In collaboration with Allan Weissman, we found that this ubiquitination was mediated by the ER-localized ubiquitin ligase HRD1. These findings indicate that serine-dependent, HRD1-mediated ubiquitination targets TCR-alpha to the ERAD pathway. Therefore, Vpu does not induce a viral-specific modification but exploits endogenous machinery for serine-dependent ubiquitination in order to downregulate CD4.
Additional Funding
- Intramural AIDS Targeted Antiviral Program (IATAP)
Publications
- Burgos PV, Mardones GA, Rojas AL, daSilva LL, Prabhu Y, Hurley JH, Bonifacino JS. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell 2010 16:425-436
- Magadán JG, Pérez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog. 2010 6:e1000869
- Pérez-Victoria FJ, Abascal-Palacios G, Tascón I, Kajava A, Magadán JG, Pioro EP, Bonifacino JS, Hierro A. Structural basis for the wobbler mouse neurodegenerative disorder caused by mutation in the Vps54 subunit of the GARP complex. Proc Natl Acad Sci U S A 2010 107:12860-5
- Ishikura S, Weissman AM, Bonifacino JS. Serine residues in the cytosolic tail of the T-cell antigen receptor alpha-chain mediate ubiquitination and endoplasmic reticulum-associated degradation of the unassembled protein. J Biol Chem. 2010 285:23916-24
- Pérez-Victoria FJ, Schindler C, Magadán JG, Mardones GA, Delevoye C, Romao M, Raposo G, Bonifacino JS. Ang2/Fat-free is a conserved subunit of the Golgi-associated Retrograde Protein (GARP) complex. Mol Biol Cell 2010 Aug 4:[Epub ahead of print]
Collaborators
- Aitor Hierro, PhD, CIC-bioGUNE, Bilbao, Spain
- Klaus Strebel, PhD, Laboratory of Molecular Microbiology, NIAID, Bethesda, MD
- Yihong Ye, PhD, Laboratory of Molecular Biology, NIDDK, Bethesda, MD
- Allan Weissman, MD, Laboratory of Protein Dynamics and Signaling, NCI, Frederick, MD
Contact
For further information, contact juan@helix.nih.gov or visit cbmp.nichd.nih.gov/sipt.


