You are here: Home > Section on Intracellular Protein Trafficking
Protein Sorting in the Endosomal-Lysosomal System

- Juan S. Bonifacino, PhD, Head, Section on Intracellular Protein Trafficking
- Rafael Mattera, PhD, Staff Scientist
- Xuefeng Ren, PhD, Research Fellow
- Shweta Jain, PhD, Postdoctoral Fellow
- Shuhei Ishikura, PhD, Postdoctoral Fellow
- Javier G. Magadán, PhD, Postdoctoral Fellow
- Yogikala Prabhu, PhD, Postdoctoral Fellow
- Christina A. Schindler, PhD, Postdoctoral Fellow
- Krishnakant Soni, PhD, Postdoctoral Fellow
- Kathryn E. Tifft, PhD, Postdoctoral Fellow
- Ginny Farias, PhD, Visiting Fellow
- Xiaoli Guo, PhD, Visiting Fellow
- Xiaolin Zhu, RN, Technician
We investigate the molecular mechanisms by which transmembrane proteins are sorted to different compartments of the endomembrane system such as endosomes, lysosomes, and a group of cell type–specific organelles known as lysosome-related organelles (e.g., melanosomes and platelet dense bodies). Sorting is mediated by recognition of signals present in the cytosolic domains of the transmembrane proteins by adaptor proteins that are components of membrane coats (e.g., clathrin coats). Among these adaptor proteins are the heterotetrameric AP-1, AP-2, AP-3, and AP-4 complexes, the monomeric GGA proteins, and the heteropentameric retromer complex. Proper sorting requires the function of additional components of the trafficking machinery that mediate vesicle tethering and fusion. Current work in our laboratory is aimed at elucidating the structure, regulation, and physiological roles of coat proteins and vesicle-tethering factors and investigating human diseases that result from genetic defects (e.g., Hermansky-Pudlak syndrome and neurodegenerative and neurodevelopmental disorders) in these proteins.
Mechanism of recognition of dileucine-based sorting signals
The AP-4 complex is the most-recently discovered and least understood of the family of AP complexes. In recent work, we found that AP-4 interacts with the cytosolic tail of the Alzheimer's disease amyloid precursor protein (APP). Disruption of the AP-4–APP interaction shifts the distribution of APP from endosomes to the trans-Golgi network (TGN) and enhances gamma-secretase–catalyzed processing of APP to the pathogenic amyloid-beta peptide. These findings establish AP-4 as a novel regulator of APP processing and trafficking and as a potential risk factor for Alzheimer's disease.
Role of the GARP complex in transport from endosomes to the Golgi complex
AP-1, AP-2, and AP-3 are clathrin-associated adaptor complexes that recognize two types of sorting signal, referred to as tyrosine-based and dileucine-based. Previous studies showed that tyrosine-based signals bind to the mu1, mu2, and mu3 subunits, whereas dileucine-based signals bind to a combination (i.e., a hemicomplex) of two subunits—gamma-sigma1, alpha-sigma2 and delta-sigma3—from the corresponding AP complexes. Structure-based mutational analyses allowed us to pinpoint the location of the binding sites for dileucine-based sorting signals fitting the DEXXXLLI consensus motif on the AP-1, AP-2, and AP-3 complexes. The location and topography of the corresponding sites are similar, although the strength and amino-acid requirements of different interactions depend on the exact sequence of the signal and the particular AP complex involved. We also demonstrated multiple AP-1 complexes resulting from combinatorial assembly of various gamma (i.e., gamma1 and gamma2) and sigma1 (i.e., sigma1A, sigma1B, and sigma1C) subunit isoforms that are encoded by different genes. The AP-1 variants bind to dileucine-based signals with marked preferences for certain sequences, implying that the variants are not functionally equivalent. Indeed, it was recently shown that mutations in sigma1A and sigma1B are the cause of two severe neurodevelopmental disorders, known as the MEDNIK and Fried syndromes, respectively. Based on our work, we hypothesize that the diseases are caused by abnormal sorting of specific cargo proteins that bear dileucine-based signals.
Sorting of the amyloid precursor protein regulated by AP-4
The AP-4 complex is distinct from other AP complexes in that it does not recognize canonical tyrosine-based and dileucine-based signals. We recently found that, instead, the mu4 subunit of AP-4 binds to an YKFFE sequence from the cytosolic tail of the Alzheimer's disease amyloid precursor protein (APP). Biochemical and X-ray crystallographic analyses revealed that the properties of the APP sequence and the location of the binding site on mu4 are distinct from those of canonical tyrosine-based signals binding to the mu subunits of other AP complexes. Two APP-like proteins, APLP1 and APLP2, bear related sequences that also interact with mu4. Disruption of the AP-4–APP interaction shifts the distribution of APP from endosomes to the trans-Golgi network and enhances gamma-secretase–catalyzed processing of APP to the pathogenic amyloid-beta peptide. Our results demonstrate that APP and AP-4 engage in a novel type of signal-adaptor interaction that mediates transport of APP from the trans-Golgi network to endosomes, thereby reducing amyloidogenic processing of the protein. AP-4 should thus be considered a novel regulator of APP processing and trafficking and a potential risk factor for Alzheimer's disease.
Role of the GARP complex in transport from endosomes to the Golgi complex
In earlier work, we found that a complex named GARP is a critical component of the molecular machinery that mediates retrograde transport of various cargo proteins, including sorting receptors, processing endopeptidases, fusogenic proteins, and bacterial and plant toxins, from endosomes to the trans-Golgi network in mammalian cells. We showed that the molecular function of this complex is to promote tethering and fusion of endosome-derived transport carriers to the trans-Golgi network. Biochemical analyses showed that the human GARP complex comprises four subunits named Vps52, Vps53, Vps54, and Ang2. Interference with any of the GARP subunits blocks the delivery of many cargos to the trans-Golgi network, leading to global defects in lysosomal function, lipid traffic, and autophagy. In collaboration with Aitor Hierro, we solved the crystal structure of a C-terminal fragment from Vps54, the first atomic structure to be solved for any part of the GARP complex. The structure revealed that that GARP is related to other multisubunit tethering complexes such as the exocyst and conserved oligomeric Golgi (COG) complex. In addition, we found that a leucine-967 to glutamine substitution in Vps54 identified in the wobbler mouse, an animal model for amyotrophic lateral sclerosis (ALS), destabilizes the protein, leading to lower levels of the GARP complex in all tissues. The motor neuron degeneration that is characteristic of this mutant mouse is therefore attributable to reduced levels of GARP and ensuing defects in retrograde transport.
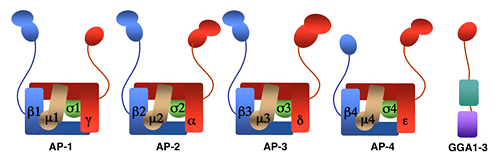
Figure 1. Structure of AP complexes and GGAs.
Mechanisms of CD4 downregulation by the Nef and Vpu proteins of HIV-1
The main goal of this project is to determine how the Vpu protein encoded in the human immunodeficiency virus-1 (HIV-1) genome downregulates the CD4 co-receptor from the surface of infected cells. Specifically, we aim to identify the host-cell factors required for downregulation, and to explain the molecular mechanisms involved.
Primate immunodeficiency viruses target helper T-cells and macrophages/monocytes through binding of the viral envelope glycoprotein to a combination of CD4 and a chemokine receptor (CCR4 or CXCR5) on the surface of the host cells. Strikingly, infection results in rapid and sustained downregulation of CD4 and, to a lesser extent, the chemokine receptors. Downregulation of these viral co-receptors prevents superinfection, promotes virion release, and interferes with the immune response, leading to the establishment of a robust infection. CD4 downregulation is so important to the life cycle of HIV-1 that two accessory proteins, Nef and Vpu, encoded in the viral genome are devoted to this task. Indeed, Nef and Vpu are critical for the progression from infection to AIDS, a fact that is best illustrated by the existence of long-term nonprogressors who are infected with HIV-1 strains bearing inactivating mutations in the genes encoding these proteins. Therefore, pharmacologic or biologic perturbation of Nef and/or Vpu has the potential to prevent the pathogenic effects of HIV-1. To date, however, this potential has not been realized mainly because Nef and Vpu have no enzymatic activity and their mechanisms of action are insufficiently understood.
In earlier work, we made substantial progress towards elucidating the mechanism of CD4 downregulation by Nef. We found that Nef connects surface CD4 to both the endocytic and lysosomal targeting machineries, leading to efficient and sustained removal of CD4 from host cells early during infection. The current project focuses on the mechanisms by which Vpu downregulates CD4 at later stages of infection. Vpu is a small transmembrane protein comprising a short luminal domain, a single transmembrane domain (TMD), and a cytosolic domain. The Vpu cytosolic domain simultaneously binds to the CD4 cytosolic tail and the SCF-beta–TrCP E3 ubiquitin ligase complex, causing ubiquitination of CD4 and its subsequent targeting for degradation by the proteasome. Our studies revealed the following novel aspects of this process: (i) degradation involves at least some components of the cellular ER-associated degradation (ERAD) machinery, including the VCP-UFD1L-NPL4 dislocase complex; (ii) CD4 ubiquitination depends not only on lysine but also on serine and threonine residues in the CD4 tail; (iii) Vpu mediates CD4 retention in the ER in addition to targeting to ERAD; and (iv) the transmembrane domain of Vpu is required for both ER retention and ERAD targeting of CD4. The multiple levels at which Vpu engages cellular quality control mechanisms underscore the importance of ensuring profound suppression of CD4 to the life cycle of HIV-1.
A surprising finding of our studies on Vpu-induced CD4 downregulation was the requirement of serine and threonine residues for CD4 ubiquitination and targeting to ERAD. To determine how common this requirement is, we examined the degradation of a prototypical ERAD substrate, the alpha subunit of the T-cell antigen receptor complex (TCR-alpha). TCR-alpha is a type I integral membrane protein that becomes ubiquitinated and targeted to ERAD when it fails to assemble into the complete TCR complex. Remarkably, TCR-alpha has a cytosolic tail of only five amino acid residues (i.e., RLWSS), none of which is the canonical ubiquitin-acceptor lysine. We found that substitution of two conserved serine residues in the cytosolic tail of TCR-alpha to alanine reduced ubiquitination, whereas placement of additional serine residues enhanced it. Moreover, replacement of the cytosolic serine residues by other ubiquitinatable residues (i.e., cysteine, threonine, or lysine) allowed ubiquitination to take place. Serine-dependent ubiquitination perfectly correlated with targeting of TCR-alpha for ERAD. We also found that this ubiquitination is mediated by the ER-localized ubiquitin ligase HRD1. These findings indicated that serine-dependent, HRD1-mediated ubiquitination targets TCR-alpha to the ERAD pathway. Thus, Vpu does not induce a viral-specific modification but exploits endogenous machinery for serine-dependent ubiquitination in order to downregulate CD4.
Additional Funding
- Intramural AIDS Targeted Antiviral Program (IATAP)
Publications
- Burgos PV, Mardones GA, Rojas AL, daSilva LL, Prabhu Y, Hurley JH, Bonifacino JS. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell 2010;16:425-436.
- Perez-Victoria FJ, Schindler C, Magadan JG, Mardones GA, Delevoye C, Romao M, Raposo G, Bonifacino JS. Ang2/fat-free is a conserved subunit of the Golgi-associated retrograde protein complex. Mol Biol Cell 2010;21:3386-3395.
- Magadán JG, Pérez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog 2010;6:e1000869.
- Ishikura S, Weissman AM, Bonifacino JS. Serine residues in the cytosolic tail of the T-cell antigen receptor alpha-chain mediate ubiquitination and endoplasmic reticulum-associated degradation of the unassembled protein. J Biol Chem 2010;285:23916-23924.
- Mattera R, Boehm M, Chaudhuri R, Prabhu Y, Bonifacino JS. Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J Biol Chem 2011;286:2022-2030.
Collaborators
- Eric Freed, PhD, HIV Drug Resistance Program, NCI, Frederick, MD
- Aitor Hierro, PhD, CIC-bioGUNE, Bilbao, Spain
- James Hurley, PhD, Laboratory of Molecular Biology, NIDDK, Bethesda, MD
- Klaus Strebel, PhD, Laboratory of Molecular Microbiology, NIAID, Bethesda, MD
- Yihong Ye, PhD, Laboratory of Molecular Biology, NIDDK, Bethesda, MD
- Allan Weissman, MD, Laboratory of Protein Dynamics and Signaling, NCI, Frederick, MD
Contact
For further information, contact juan@helix.nih.gov or visit cbmp.nichd.nih.gov/sipt.

