You are here: Home > Section on Membrane Biology
Membrane Fusion Mediated by Protein Fusogens

- Leonid V. Chernomordik, PhD, Head, Section on Membrane Biology
- Eugenia Leikina, DVM, Senior Research Assistant
- Kamram Melikov, PhD, Staff Scientist
- Elena Zaitseva, PhD, Research Fellow
- Samristha Sanyal, PhD, Visiting Fellow
- Sung-Tae Yang, PhD, Visiting Fellow
- Margarita S. Popova, MB, NIH Summer Student Program
Disparate membrane remodeling reactions are tightly controlled by protein machinery but are also dependent on the lipid composition of the membranes. Whereas each kind of protein has its own individual personality, membrane lipid bilayers have rather general properties manifested by their resistance to disruption and bending. Our long-term goal is to understand how proteins remodel membrane lipid bilayers in important cell biology processes. The starting point of our analysis is a consideration of the physical factors that determine the tendency of the membrane bilayers to change their topology. We expect that the analysis of the molecular mechanisms of important and diverse membrane rearrangements will bring about new ways of controlling them and clarify the generality of emerging mechanistic insights. In recent studies we have focused on mechanisms of influenza hemagglutinin–mediated fusion and late stages of cell-to-cell fusion.
The final conformation of the complete ectodomain of the HA2 subunit of influenza hemagglutinin can by itself drive low pH–dependent fusion.
Click image to enlarge.
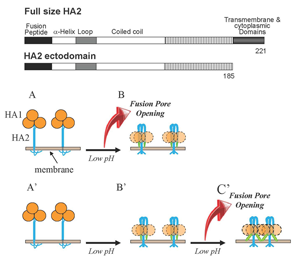
Figure 1. Schematic diagram depicting two mechanisms by which HA restructuring at low pH may be coupled to membrane fusion
HA1 subunits in HA homotrimers are shown as orange circles. HA2 subunits responsible for HA–mediated fusion are shown as blue shapes. Fusion peptides—amphiphilic amino terminal regions of HA2 exposed only in low pH forms of the protein—are shown as green shapes and membranes are shown in grey-brown. For the sake of simplicity, one of two fusing membranes is not shown. A, B. Fusion can be driven by the conformational energy released in HA2 restructuring from initial neutral-pH form to the low-pH hairpin form. In this scenario, the hairpin conformation represents the "discharged" post-fusion form of HA2. A', B', C'. Low pH–triggered restructuring of HA2 produces hairpin form of the protein, and this form mediates low pH–dependent fusion. In this hypothetical scenario, interactions between adjacent HA2 molecules and between HA2 and membranes may provide the energy for membrane remodeling. Our finding that hairpin conformations of HA2 ectodomain form fusion pores in a low pH–dependent manner substantiates the pathway A'→B'→C'. Insert shows schematic diagrams of the full-length HA2 subunit (residues 1–221) and HA2 ectodomain (residues 1–185) lacking only the transmembrane and short cytoplasmic domains.
The molecular mechanisms of fusion of lipid bilayers in remodeling of intracellular membranes, in entry of enveloped viruses, and in cell-to-cell fusion during development remain to be clarified even for the best characterized examples of biological fusions such as fusion mediated by the influenza virus hemagglutinin (HA). HA–mediated fusion between the viral envelope and the endosomal membrane delivers viral RNA into cytosol. In the initial conformation of HA, its fusogenic subunit, the transmembrane protein HA2, is locked in a metastable conformation by the receptor-binding HA1 subunit of HA. Acidification in the endosome triggers HA2 refolding towards the final lowest-energy conformation. To examine whether the fusion process is driven by this final conformation or, as often suggested, by the energy released by protein restructuring (Figure 1B and 1A, respectively), we explored structural properties as well as the fusogenic activity of the full-sized trimeric HA2(1–185) (referred to as HA2*) that presents the final conformation of the HA2 ectodomain. We found that HA2* mediates fusion between lipid bilayers and between biological membranes. Two mutations known to inhibit HA–mediated fusion strongly inhibit the fusogenic activity of HA2*. At surface densities similar to those of HA in the influenza virus particle, HA2* forms small fusion pores but does not expand them. Interestingly, HA2* mediates lipid and content mixing only after application of acidic pH. The pH dependence of the HA2*–mediated fusion is in the range of those observed for other pH–dependent functions of the full-sized HA of the X-31 strain of influenza virus. The ability of HA2* to mediate low pH–dependent fusion suggests that there must be functionally important low pH–dependent interactions between the final conformations of HA2 subunits and/or between these conformations and the membranes. Indeed, acidic pH is known to affect conformation and self-association of membrane-inserted synthetic peptides mimicking the HA2 N-terminal fusion peptide domains of HA. Specific mechanisms by which the complete ectodomain HA2 fuses both model and biological membranes, as well as the role of the fusogenic properties of the final conformation of HA2 subunit in the context of fusion mediated by full-sized HA, remain to be established. However, our findings suggest that formation of the hairpin conformation of HA2, often referred to as a post-fusion conformation, does not signify the end of functionally important HA– and membrane-restructuring but rather represents the beginning of the key fusion stage (Figure 1B). Restructuring of protein fusogens into a hairpin-like final structure under fusion conditions is a strikingly conserved mechanistic motif shared by very diverse viruses. Thus, the question whether these hairpin conformations represent discharged post-fusion forms of the proteins or their functional fusogenic forms is not limited solely to fusion mediated by HA.
Intracellular curvature-generating proteins in cell-to-cell fusion
Click image to enlarge.
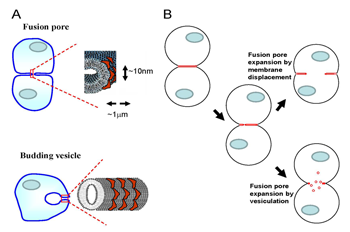
Figure 2. Cytosolic membrane curvature–generating proteins can expand fusion pore(s) by facilitating either displacement of membrane material towards the periphery of the contact zone or vesiculation of the contact zone.
A. The degree of membrane bending at the semicylindrical rim of the fusion pore connecting two cells is similar to that of intracellular membrane structures (for instance, endocytic vesicles) formed by curvature-generating proteins (red shapes). B. Curvature-generating proteins can promote late stages of syncytium formation within either of two alternative mechanisms of fusion-pore expansion: membrane displacement or vesiculation.
Early stages of cell-to-cell fusion in developmental processes, such as fertilization, muscle and bone formation, and in pathological processes, such as viral infections and carcinogenesis, culminate in the opening of nanometer-sized fusion pores that connect the volumes of two cells. The mechanisms driving subsequent expansion of the fusion pore(s) to a micrometer-sized lumen that permits complete coalescence of cytoplasms are poorly understood. The membrane bilayer at the edge of the pore growing within the tight contact zone remains strongly curved and hence accumulates the elastic energy of bending. The degree of membrane bending at the pore rim is similar to that of intracellular membrane structures such as membrane tubules and endocytic vesicles (Figure 2A). We hypothesized that the cytosolic proteins involved in cell-controlled bending of intracellular membranes accumulate at the fusion pore rim, lower its energy, and thus promote pore expansion and syncytium formation. We explored whether changes in the activity of the proteins that bend intracellular membranes affect cell fusion initiated by protein fusogens of influenza virus and baculovirus. We raised the intracellular concentration of curvature-generating proteins in cells by either expressing or microinjecting the ENTH domain of Epsin or by expressing the GRAF1 BAR domain or FCHo2 F-BAR domain. Each treatment promoted syncytium formation. The extent of cell fusion was also influenced by treatments targeting the function of another curvature-generating protein, dynamin. Cell membrane–permeable inhibitors of dynamin GTPase blocked expansion of fusion pores, and dominant negative mutants of dynamin influenced the syncytium formation extents. We also found that syncytium formation is inhibited by reagents lowering the content and accessibility of phosphatidylinositol(4,5)bisphosphate, an important regulator of intracellular membrane remodeling. Our findings indicate that fusion pore expansion at late stages of cell-to-cell fusion is mediated, directly or indirectly, by intracellular membrane-shaping proteins. However, several important questions remain. First, the mechanisms underlying the dependence on curvature-generating proteins are yet to be clarified. The proteins can either directly facilitate fusion pore expansion by accumulating at the pore edge and lowering its energy or promote vesiculation of the membrane junction at the edge of the fusion pores (Figure 2B). A better understanding of the mechanism and cell machinery responsible for driving fusion pore expansion in cell-to-cell fusion will bring about new ways of controlling fusion in development and in pathological conditions. Second, knowing how fusion pore expansion depends on cell machinery can also be important for understanding why transient nanotubular connections between plasma membranes of some cells do not expand to yield multinucleated cells. Our work also emphasizes an interesting overlap between proteins controlling late stages of cell-to-cell fusion and proteins that drive the oppositely directed process of membrane remodeling—fission of one cell membrane into two. Curvature-generating proteins that we found to influence syncytium formation are essential components of various endocytic pathways. Further elucidation of the overlap between the protein players involved in the processes that unite and divide biological membranes is important for finding shared mechanistic principles underlying fusion and fission.
Additional Funding
- NIAID's 2009-2011 Trans-NIH Biodefense Program
- NIH's Intramural AIDS Targeted Antiviral Program (IATAP) 2011-2012
Publications
- Kozlov MM, McMahon HT, Chernomordik LV. Protein-driven membrane stresses in fusion and fission. Trends Biochem Sci 2010;35:699-706.
- Zaitseva EM, Yang S, Melikov K, Pourmal SA, Chernomordik LV. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog 2010;6:e1001131.
- Yang S, Zaitseva EM, Chernomordik LV, Melikov K. Cell penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid. Biophys J 2010;99:2525-2533.
- Rafikova ER, Melikov K, Chernomordik LV. Cytosol dependent membrane fusion in ER, nuclear envelope and nuclear pore assembly: biological implications. Nucleus 2010;1:487–491.
- Kim CS, Epand RF, Leikina E, Epand RM, Chernomordik LV. The final conformation of the complete ectodomain of the HA2 subunit of influenza hemagglutinin can by itself drive low pH-dependent fusion. J Biol Chem 2011;286:13226-34.
Collaborators
- Michael M. Kozlov, PhD, DHabil, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- Tamas Balla, MD, PhD, D(Med)Sc, Program in Physical Biology, NICHD, Bethesda, MD
- Raquel Epand, PhD, McMaster University, Hamilton, Ontario, Canada
- Richard Epand, PhD, McMaster University, Hamilton, Ontario, Canada
- William Mike Henne, PhD, Cornell University, Ithaca, NY
- Chang Sup Kim, PhD, Hanbat National University, Daejeon, South Korea
- Ralf Langen, PhD, Zilkha Neurogenetic Institute, University of Southern California, Los Angeles, CA
- Harvey T. McMahon, PhD, MRC Laboratory of Molecular Biology, Cambridge, UK
- Jean-Philippe Richard, PhD, The Johns Hopkins School of Medicine, Baltimore, MD
Contact
For more information, email chernoml@mail.nih.gov.

