You are here: Home > Unit on Reproductive Endocrinology and Infertility
Reproductive Endocrinology and Infertility

- James Segars, MD, Head, Unit on Reproductive Endocrinology and Infertility
- Alicia Armstrong, MD, Associate Fellowship Program Director, Reproductive Endocrinology and Infertility
- Pamela Stratton, MD, Head, Gynecology Consult Service
- William H. Catherino, MD, PhD, Special Volunteer
- Hisashi Koide, MD, PhD, Visiting Fellow
- John Norian, MD, Clinical Fellow, Reproductive Endocrinology and Infertility
- Marcy Maguire, MD, Clinical Fellow, Reproductive Endocrinology and Infertility
- Kimberly M. Baig, BS, MD/PhD Student
- Xiaoxiao C. Guo, Student Fellow
- Aliye Runyan, BS, HHMI Fellow
Uterine leiomyomas
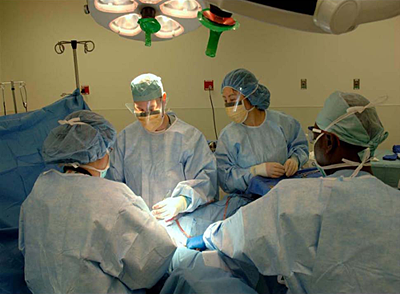
Click image to enlarge.
Figure 1. Surgeons in the Unit remove a symptomatic uterine leiomyoma.
The Unit on Reproductive Endocrinology conducts clinical, translational, and basic science studies of uterine leiomyoma.
Fifty percent of women in the United States develop uterine fibroids during their reproductive years, making the condition the most prevalent reproductive disorder of women. Despite its prevalence, the condition remains poorly understood. One prominent feature of uterine fibroids is that the cells within the tumors produce a disordered and excessive extracellular matrix (ECM). In earlier work, we examined the characteristics of the cells that might lead to production of this excessive and fibrotic ECM and obtained evidence that mechanical signaling—a method of cell communication and activation—was altered in cells within a fibroid. During the past year, we examined surgical specimens and immortalized leiomyoma and myometrial cells and indeed found that mechanical signaling was attenuated in leiomyoma cells compared with myometrial cells. In addition, the visco-elastic properties of leiomyoma contributed substantially to the stiff, rubbery nature of the tumors. The findings raises the possibility that mechanical signaling may contribute to leiomyoma growth. Additionally, we completed a collaborative clinical trial with Bradford Wood and Aradhana Venkatesan of MRI–guided high-frequency ultrasound (HIFU) for the non-surgical treatment of uterine fibroids. In the coming year, we plan to continue our study of mechanical signaling in fibroid cells and to initiate a second study with HIFU for the non-surgical treatment of uterine fibroids.
Role of BRX (also known as AKAP13) in osmotic stress, inflammation, and development
Our previous studies of the gene BRX (now known as AKAP13), with we cloned, indicated that the gene encoded a large Rho-GEF proto-oncoprotein that is involved in estrogen and glucocorticoid receptor activation. In a recent collaborative study with Tomoshige Kino using mice haploinsufficient for BRX, we found that BRX was critical to the response of lymphocytes to osmotic stress through a pathway involving the nuclear factor of activated T cells 5 (NFAT5) and JIP4. In the past year, we reported that mice deficient for both alleles of the gene (Brx−/− mice) died in utero because of a failure of heart development. Our studies revealed that the BRX gene product coordinates G as and Rho signaling with an essential transcription program in developing cardiomyocytes of mice, involving myocyte enhancer factor 2 C (MEF2C). In the past year, we also developed a conditional gene-targeting strategy using the Cre-Lox system in mice. In the coming year, we plan to examine the consequence(s) of haploinsufficiency for AKAP13 and to begin analysis of the Cre-Lox AKAP13 tissue–specific deletion in mice.
Assessment and preservation of ovarian function in women and girls undergoing cancer treatment
Because of effective chemotherapy for cancers, many young girls and women are now able to survive cancer, but find that reproductive function is impaired. In the past year, we concluded studies of a murine model of medical treatment prior to chemotherapy in an effort to explore the mechanisms responsible for preservation of ovarian function with gonadotropin releasing hormone (GnRH) antagonist therapy. Previously, we found that pre-treatment of mice with a GnRH-antagonist reduced ovarian damage associated with the chemotherapeutic agent cyclophosphamide. The use of a GnRH antagonist for this purpose has the possible clinical benefit of not causing the "flare effect" that is observed with GnRH agonist therapy. In the past year, we examined the mechanism(s) responsible for the protective effect of GnRH antagonist therapy prior to chemotherapy and reported that the protective effect of GnRH was associated with a reduction in programmed cell death (apoptosis) in ovarian follicles. In the coming year, we plan to continue our clinical study of ovarian function in pediatric cancer survivors.
Endometriosis and chronic pelvic pain
The group led by Pamela Stratton examined the relationship between patient-reported, physician-diagnosed comorbid conditions and endometriosis in 4,331 women with endometriosis. The study found that women with endometriosis had a higher prevalence of recurrent upper respiratory or vaginal infections, melanoma, and ovarian cancer that the general population. In another study reported last year, the group examined whether pain location was related to lesion location in women with chronic pelvic pain and endometriosis. The investigators found that lesion depth, disease severity (burden), and the number of lesions were not associated with pain location. Only painful urination (dysuria) and midline abdominal pain were associated with location of superfical endometriosis lesions. In the coming year, the relationship between chronic pelvic pain and endometriosis will be compared in three cohorts of women: women with chronic pain and endometriosis, those with chronic pelvic pain but no evidence of endometriosis, and healthy volunteers. The goals of that clinical trial are to characterize (i) the relationship between chronic pelvic pain, endometriosis, central nervous system sensitization, and myofascial dysfunction; (ii) the HPA axis and to correlate changes in ACTH and cortisol response with depression and anxiety scores; and (iii) the relationship between nerves in the endometrium and endometriosis lesions and chronic pelvic pain.
Infertility and reproductive health disparities
We continued to conduct studies on infertility and reproductive health disparities. Previously, we examined health disparities in reproductive outcome in women undergoing assisted reproduction treatments. This clinical study indirectly examined the hypothesis that lower pregnancy outcomes observed in African-American women than in Caucasian women are a result of altered endometrial responses possibly due to higher levels of estrogen during the ovarian stimulation. Consistent with that hypothesis, when the endometrium was prepared similarly for embryo transfer, no ethnic differences in pregnancy rates were observed. In the past year, we examined factors such as cost in utilization of reproductive technologies by minority women. We found that economics and access to care influences use by African-American women but not by Hispanic women. Furthermore, despite increased use of assisted reproductive technologies by African American women, outcomes were worse than for Caucasian women. The results suggest that improved access to care may not translate into improved outcomes in some ethnic groups.
Additional Funding
- Office of Dietary Supplements0
Publications
- Mayers CM, Wadell J, McLean K, Venere M, Malik M, Shibata T, Driggers PH, Kino T, Guo XC, Koide H, Gorivodsky M, Grinberg A, Mukhopadhyay M, Abu-Asab M, Westphal H, Segars JH. The RHO-guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J Biol Chem 2010;285:12344-54.
- Taylor JE, Miller BT, Gray KD, Scott RT Jr, Catherino WH, Segars JH. The mechanism responsible for the supraphysiologic gonadotropin surge in females treated with gonadotropin-releasing hormone (GnRH) agonist and primed with GnRH antagonist. Fertility and Sterility 2010;93:1668-75.
- Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol 2011;118:223-30.
- Browne HN, Moon KS, Mumford SL, Schisterman EF, Decherney AH, Segars JH, Armstrong AY. Is anti-Müllerian hormone a marker of acute cyclophosphamide-induced ovarian follicular destruction in mice pretreated with cetrorelix?. Fertil Steril 2011;96:180-86.
- McCarthy-Keith DM, Malik M, Britten J, Segars J, Catherino WH. Gonadotropin-releasing hormone agonist increases expression of osmotic response genes in leiomyoma cells. Fertil Steril 2011;95:2383-7.
Collaborators
- Ayman Al-Hendy, MD, PhD, Meharry Medical College, Nashville, TN
- Paul Driggers, PhD, Uterine Fibroid Tissue Bank, USUHS-NICHD, Bethesda, MD
- Tomoshige Kino, PhD, Program in Reproductive and Adult Endocrinology, NICHD, Bethesda, MD
- Phyllis Leppert, MD PhD, Duke University, Durham, NC
- Lawrence Nelson, MD, Program in Reproductive and Adult Endocrinology, NICHD, Bethesda, MD
- Lynnette Nieman, MD, Program in Reproductive and Adult Endocrinology, NICHD, Bethesda, MD
- Steven Z. Pavletic, MD, Experimental Transplantation and Immunology Branch, NCI, Bethesda, MD
- Mark Payson, MD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Pamela G. Robey, PhD, Craniofacial and Skeletal Diseases Branch, NIDCR, NIH, Bethesda, MD
- Ninet Sinaii, MPH, PhD, Office of the Deputy Director for Clinical Care, Clinical Center, NIH, Bethesda, MD
- Rocky Tuan, PhD, Cartilage Biology and Orthopaedics Branch, NIAMS, Bethesda, MD
- Aradhana Venkatesan, MD, Radiology and Imaging Sciences, NIH Clinical Center, Bethesda, MD
- Heiner Westphal, MD, Program in Genomics of Differentiation, NICHD, Bethesda, MD
- Bradford J. Wood, MD, Center for Interventional Oncology, NIH Clinical Center, Bethesda, MD
Contact
For more information, email segarsj@mail.nih.gov.

