You are here: Home > Section on Molecular Morphogenesis
Thyroid Hormone Regulation of Vertebrate Postembryonic Development

- Yun-Bo Shi, PhD, Head, Section on Molecular Morphogenesis
- Liezhen Fu, PhD, Staff Scientist
- Thomas Miller, PhD, Postdoctoral Intramural Research Training Award Fellow
- Kenta Fujimoto, PhD, Visiting Fellow
- Fiona Mitchell, PhD, Visiting Fellow
- Julia Rodiger, PhD, Visiting Fellow
- Luan Wen, PhD, Visiting Fellow
- Yu Zhang, PhD, Visiting Fellow
- Nga Luu, MS, Biologist
This laboratory investigates the molecular mechanisms of thyroid hormone function during postembryonic development. The main model used is the metamorphosis of the amphibians Xenopus laevis, a species well studied as a developmental model, and Xenopus tropicalis, a recently developed model system, whose shorter life cycle and sequenced genome offer some unique advantages. The control of the developmental process by thyroid hormone (TH) offers a unique paradigm for the study of gene function in postembryonic organ development. During metamorphosis, various organs undergo vastly different changes. Some, like the tail, undergo complete resorption, while others, such as the limb, develop de novo. The majority of the larval organs persist through metamorphosis but are dramatically remodeled to function in a frog. For example, tadpole intestine in Xenopus is a simple tubular structure consisting primarily of a single layer of larval epithelial cells. During metamorphosis, it is transformed into an organ with a multiply folded adult epithelium surrounded by elaborate connective tissue and muscles. The process occurs through specific larval epithelial cell death and de novo development of adult epithelial stem cells followed by their proliferation and differentiation. The wealth of knowledge from past research and the ability to manipulate amphibian metamorphosis both in vivo, by using transgenesis or hormone treatment of whole animals, and in vitro in organ cultures offer excellent opportunities to study the developmental function of TH receptors (TRs) and the underlying mechanisms in vivo, on the one hand, and, on the other, to identify and functionally characterize genes critical for postembryonic organ development in vertebrates. Our studies have revealed likely conserved mechanisms in adult intestinal stem cell development in vertebrates, prompting us to adapt the mouse model, which complements the amphibian system, for gene knockout studies.
Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during intestinal metamorphosis.
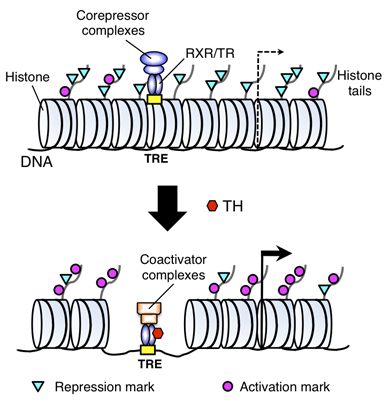
Figure 1. A model for gene regulation by TR
TH functions by regulating gene transcription through TH receptors (TRs). In the absence of TH (as in the premetamorphic tadpole), TR forms heterodimers with RXR (9-cis retinoic acid receptor), and the heterodimer binds to the TH response elements (TREs) in the target genes to repress their expression by recruiting corepressor complexes containing histone deacetylases. When TH is present, the corepressor complexes are released, and liganded TR/RXR recruits coactivator complexes containing histone acetyltransferases and histone methyltransferases such as PRMT1 (protein arginine methyltransferase 1). The coactivator complexes modify histones or cause the removal of nucleosomes, leading to the activation of gene expression.
We previously showed that the heterodimers between TR and RXR (9-cis retinoic acid receptor) have dual functions during frog development. In premetamorphic tadpoles, the receptors repress gene expression in the absence of TH to prevent metamorphosis, thus ensuring a proper tadpole growth period. When TH is present either from endogenous synthesis during development or by adding it exogenously to the rearing water of premetamorphic tadpoles, TR/RXR heterodimers activate TH–inducible genes to initiate metamorphosis. To investigate how the heterodimers regulate gene expression in vivo, we analyzed histone modifications at TH target genes during intestinal metamorphosis in Xenopus tropicalis, a process that involves total apoptotic degeneration of the simple larval epithelium and de novo development of the adult epithelial stem cells, followed by the cells' proliferation and differentiation into the complex adult epithelium. We demonstrated for the first time in vivo during vertebrate development that TR induces the removal of core histones at the promoter region (Figure 1) and the recruitment of RNA polymerase. Furthermore, several histone activation and repression markers were defined based on correlations with mRNA levels in cell cultures. Most, but not all, correlate with gene expression induced by liganded TR during development, suggesting that tissue and developmental context influence the role of histone modifications in gene regulation. Our findings provide important mechanistic insights into how chromatin remodeling affects developmental gene regulation in vivo.
Histone H3K79 methyltransferase Dot1L is directly activated by the thyroid hormone receptor during Xenopus metamorphosis.
To identify the direct target genes of TR during metamorphosis, we carried out a ChIP (chromatin immunoprecipitation)-on-chip analysis of the intestine by using a set of microarray chips covering an 8 kb region flanking each putative promoter of 17,000 Xenopus tropicalis genes to look for genes bound by TR (unpublished observation). While the ChIP-on-chip data were very preliminary, it was of interest that one of the putative target genes thus identified corresponded to the Xenopus tropicalis homologue of the mammalian Dot1L gene, which encodes the only histone methyltransferase capable of methylating H3K79. We showed that, in Xenopus tropicalis, Dot1L was directly regulated by TR via binding to a TH response element in the promoter region during metamorphosis. We further showed that Dot1L expression in both the intestine and tail correlated with the transformation of the organs. Given our previous finding that methylation of histone H3 lysine (K) 79 in the promoter regions of TR target genes is enhanced upon activation of TH–inducible genes (see above), our findings suggest that TR activates Dot1L, which in turn participates in metamorphosis through a positive feedback to enhance H3K79 methylation and gene activation by liganded TR.
Direct activation of Xenopus iodotyrosine deiodinase by the thyroid hormone receptor in the remodeling intestine during amphibian metamorphosis
TH production in the thyroid involves incorporating inorganic iodide into thyroglobulin. Expression of iodotyrosine deiodinase (IYD, also known as iodotyrosine dehalogenase 1 [DEHAL1]) in the thyroid gland ensures efficient recycling of iodine from the byproducts of TH biosynthesis: 3′-monoiodotyrosine and 3′,5′-diiodotyrosine. Interestingly, IYD is known to be expressed in other organs in adult mammals, suggesting that iodine recycling occurs outside the thyroid. However, the developmental role of iodine recycling has yet to be investigated. Interestingly, our preliminary ChIP-on-chip study also revealed that Xenopus tropicalis IYD gene is bound by TR in the intestine. Furthermore, we showed that IYD expression is strongly upregulated by TH during metamorphosis in the intestine but not in the tail. We further demonstrated that this induction is one of the earliest events during intestinal metamorphosis, with IYD directly activated through the binding of liganded TR to a TH–response element in the IYD promoter region. As iodide is mainly taken up from the diet in the intestine, and the tadpole stops feeding during metamorphosis when the intestine is being remodeled, our findings suggest that IYD transcription is activated by liganded TR early during intestinal remodeling to ensure efficient iodine recycling at the climax of metamorphosis, when highest levels of TH are needed for proper transformations of various organs.
Epithelial-connective tissue interactions induced by TR are essential for adult stem cell development in the Xenopus laevis intestine.
We previously showed that adult intestinal stem cells originate from differentiated larval epithelial cells in the Xenopus laevis intestine. To determine whether TH signaling in the epithelium alone is sufficient to induce adult stem cells, we performed recombinant tissue culture experiments by using transgenic Xenopus laevis tadpoles that express a dominant positive TH receptor (dpTR) under the control of a heat shock promoter, enabling us to specifically activate TH signaling in selective tissues in the intestine. Our results indicate that, while adult progenitor cells may be induced by TH action in the larval epithelium, TR–mediated gene expression in the surrounding tissues, most likely the connective tissue, is required for such tissues to develop into adult stem cells, suggesting the importance of TH–inducible epithelial-connective tissue interactions in establishing the adult stem cell niche.
To investigate the underlying mechanism, we have been studying the signaling processes that may affect cell-cell interactions. We showed previously that, during Xenopus laevis metamorphosis, Sonic hedgehog (Shh) is directly induced by TH at the transcription level—one of the earliest events during intestinal remodeling. However, regulation of other components of the signaling pathway remains to be analyzed. We analyzed the spatiotemporal expression of the Shh receptors Patched-1 (Ptc-1) and Smoothened (Smo) as well as Shh's downstream transcription factors Gli1, Gli2, and Gli3 during natural and TH–induced intestinal remodeling. We showed that all genes examined were transiently up-regulated in mesenchymal tissues during intestinal metamorphosis. Interestingly, in the presence of protein synthesis inhibitors, only Gli2 was induced by TH, suggesting that Gli2 is a direct TH–response gene, while the other factors are likely indirect ones. Furthermore, we demonstrated in the organ culture experiment that overexpression of Shh enhances the expression of Ptc-1, Smo, and the genes encoding the Gli factors even in the absence of TH, indicating that Shh regulates its own pathway components during intestinal remodeling. These findings indicate that Shh, expressed in the epithelium, regulates mesenchymal tissue development, which in turn facilitates the development of the adult intestinal stem cells during metamorphosis.
Thyroid hormone activates protein arginine methyltransferase 1 expression by directly inducing c-Myc transcription during Xenopus intestinal stem cell development.
We previously showed that protein arginine methyltransferase 1 (PRMT1) is upregulated in and required for adult intestinal stem cells during metamorphosis and that this role is evolutionarily conserved in vertebrates. PRMT1 upregulation is the earliest known molecular event for developing stem cells and is also observed during zebrafish and mouse adult intestinal stem cell development. To analyze how PRMT1 is specifically upregulated during the formation of the adult intestinal stem cells, we cloned the Xenopus PRMT1 promoter and characterized it in CaCo-2 cells, a human cell line with intestinal stem cell characteristics. Through a series of deletion and mutational analyses, we showed that the stem cell–associated transcription factor c-Myc could bind to an evolutionarily conserved site in the first intron to activate the promoter. Furthermore, we demonstrated that, during metamorphosis, both c-Myc and PRMT1 were highly upregulated specifically in the remodeling intestine, but not in the resorbing tail, and that c-Myc was induced by TH prior to PRMT1 upregulation. In addition, we showed that TH directly activated the c-Myc gene during metamorphosis in the intestine via binding of TH receptor to the c-Myc promoter. These results suggest that TH induces c-Myc transcription directly in the intestine and that c-Myc in turn activates PRMT1 expression. Such a gene regulation cascade would be important for controlling intestinal stem cell development.
Publications
- Matsuura K, Fujimoto K, Fu L, Shi Y-B. Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development. Endocrinology 2012;153:961-972.
- Matsuura K, Fujimoto K, Das B, Fu L, Lu CD, Shi Y-B. Histone H3K79 methyltransferase Dot1L is directly activated by thyroid hormone receptor during Xenopus metamorphosis. Cell Biosci 2012;2:25(1-10).
- Fujimoto K, Matsuura K, Das B, Fu L, Shi Y-B. Direct activation of Xenopus iodotyrosine deiodinase by thyroid hormone receptor in the remodeling intestine during amphibian metamorphosis. Endocrinology 2012;153:5082-5089.
- Hasebe T, Buchholz DR, Shi Y-B, Ishizuya-Oka A. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells 2011;29:154-161.
- Fujimoto K, Matsuura K, Hu-Wang E, Lu R, Shi Y-B. Thyroid hormone activates protein arginine methyltransferase 1 expression by directly inducing c-Myc transcription during Xenopus intestinal stem cell development. J Biol Chem 2012;287:10039-10050.
Collaborator
- Michael Bustin, PhD, Center for Cancer Research, NCI, Bethesda, MD
- Chuxia Deng, PhD, Genetics of Development and Disease Branch, NIDDK, Bethesda, MD
- Atsuko Ishizuya-Oka, PhD, Nippon Medical School, Tokyo, Japan
- Peter Taylor, PhD, University of Dundee, Dundee, UK
Contact
For more information, email shi@helix.nih.gov or visit smm.nichd.nih.gov.

