You are here: Home > Unit on Cellular Communication
Mechanisms of Cellular Communication During Development

- Mihaela Serpe, PhD, Head, Unit on Cellular Communication
- Mikolaj Sulkowski, PhD, Postdoctoral Fellow
- Young-Jun Kim, PhD, Visiting Fellow
- Cathy Ramos, PhD, Visiting Fellow
- Oghomwen Igiesuorobo, BS, Postbaccalaureate Fellow
- Peter Nguyen, Biological Laboratory Technician
The purpose of our research is to elucidate molecular mechanisms that regulate cell-cell communication during development. We focus on two related questions: how tissues are patterned and correctly connected by long-range signals; and how cells structures and functions are coordinated at short-range with those of their neighbors. TGF-beta factors modulate long-range signaling during patterning but also ensure short-range communication at specialized cell-cell interaction zones, for example at the neuromuscular junction (NMJ). We use the Drosophila model system and a variety of molecular and biochemical approaches to study genes that modulate the function of TGF-beta–type signals. Highly conserved throughout the animal kingdom, TGF-beta growth and differentiation factors have the ability to function as morphogens, that is, to specify cell fate in a concentration-dependent manner. In addition, the signaling factors provide a mechanism for coupling a cell to its neighbors and to its environment, so that the cell has the necessary plasticity to respond appropriately to changes around it, in its environment, or even to its own state.
TGF-beta–type signals are also used to modulate growth, development, and homeostasis at the Drosophila NMJ, a glutamatergic synapse similar in structure and function to vertebrate central synapses. In flies, each NMJ is unique and identifiable, and synapses are large and accessible for electrophysiological and optical analysis, making the Drosophila NMJ a favorite genetic system in which to study synapse development. The subunits that form the glutamate-gated ion channels (iGluRs) are known and relatively well studied. However, the mechanisms that control iGluRs clustering and stabilization at the postsynaptic densities (PSDs) remain a mystery. We discovered a novel, protein, Neto (Neuropillin and Tolloid-like), the first non-channel protein to be discovered that is essential for functional receptors. Our findings provide an entry point to understand the molecular mechanisms of synapse development.
Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction.
Click image to enlarge.
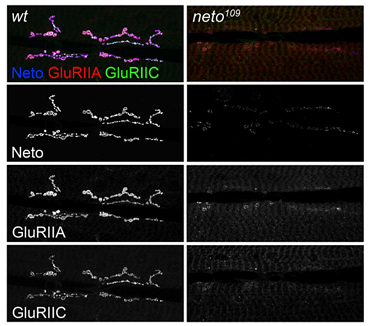
Figure 1. neto mutants exhibit defects in targeting and stabilization of glutamate receptors at the NMJ.
Localization of two different receptor subunits (GluRIIA and GluRIIC) in wt and neto hypomorphs. The images are taken at the same confocal settings to capture the dramatic redistribution of receptors from synaptic to extrajunctional locations at low Neto levels.
Synapse development is a highly orchestrated process that requires bidirectional communication between two synaptic partners across the synaptic cleft. Before a muscle is innervated, low levels of iGluRs are present diffusely in the muscle membrane at the fly NMJ. Once the motor neuron growth cone arrives at its target muscle, iGluRs begin to concentrate at the synaptic cleft. The mechanism by which neuronal arrival regulates postsynaptic clustering so far remains mysterious. Live studies demonstrated that receptor clusters are immobilized at PSDs that grow to a limited, mature size. We are interested in how iGluRs traffic to the synapse and how they form stable clusters at the mature PSDs.
Fly iGluR subunits have relatively short intracellular domains and no known extracellular binding partner. Several mechanisms regulating the abundance of synaptic glutamate receptors have been described previously, but none appear to be essential for localizing iGluRs. Possible clues to the mechanisms of trafficking and clustering of iGluRs at the fly NMJ could be provided by the emerging families of auxiliary subunits. Recent discoveries in vertebrates revealed the extraordinary diversity and richness that auxiliary subunits impart to vertebrate iGluR function. Auxiliary subunits are transmembrane proteins that avidly and selectively bind to mature iGluRs and form stable complexes at the cell surface. They can modulate the functional characteristics of iGluRs and may mediate surface trafficking and/or targeting to specific subcellular compartments. It has been reported that several Drosophila genes encode auxiliary subunits, but none have yet been characterized.
We characterized Neto as a novel molecule that directly binds to iGluR complexes and is an absolute requirement for clustering of the receptor complexes at the onset of synaptogenesis. Drosophila Neto has two vertebrate homologues called Neto-1 and Neto-2, which were recently shown to modulate the physiological properties of selective kainate-type receptors. Neto1/Neto2 double-knockout mice have defects in long-term potentiation and learning and memory but are nevertheless viable, while the Drosophila neto mutants we generated are homozygous lethal; they die as paralyzed embryos and lack any body wall peristalsis or hatching movements. Muscle expression of Neto rescued the paralyzed neto-null embryos to viable, fertile adults, indicating that Neto activity is essential in striated muscle.
In striated muscle, we found that Neto is concentrated at the NMJ and that it co-localizes with the iGluRs at the PSDs in puncta juxtaposing the active zones. The iGluRs of the Drosophila NMJ are hetero-tetrameric complexes composed of three essential subunits—GluRIIC, GluRIID, and GluRIIE—and either GluRIIA or GluRIIB. Embryos lacking any of the essential subunits, or GluR-IIA and -IIB together, are paralyzed and cannot hatch into the larval stages. The essential subunits are required not only for viability but also for the clustering of the other iGluRs at the NMJ. The similarities in subcellular localization and loss-of-function phenotype of Neto and iGluRs raised the possibility that Neto may be important for iGluRs clustering at the NMJ.
First, using an allelic series of neto mutations, we found that neto loss-of-function phenotypes parallel the loss-of-function defects described for iGluR complexes. As already mentioned, neto-null embryos lack any body wall peristalsis and hatching movements and have no detectable iGluR clusters at the NMJ. To understand how Neto controls the clustering of iGluRs, we explored the timing of Neto clustering at the NMJ by using live imaging of embryos with muscle-expressed Neto-eGFP. Neto accumulates and clusters at the NMJ as early as 14 hours after egg laying, when the iGluRs begin to accumulate and cluster at the synapse. Most important, Neto-positive puncta could not be detected in gluRIID−/−-null embryos. Thus, Neto and iGluRs depend on each other for clustering at the NMJ. Simply stated, Neto functions as a non-channel, essential subunit of the iGluR complexes.
Second, animals with suboptimal Neto levels have severe defects in postsynaptic localization of iGluRs (Figure 1). In neto hypomorphic mutant larvae (neto109), the receptor protein levels were not changed, as determined by Western blots, but the iGluRs immunorectivities were shifted from postsynaptic to extrajunctional locations. The sparse iGluR clusters in Neto-deprived NMJ synapses always colocalize with the remaining Neto clustes, indicating that the complexes must contain Neto and iGluRs in order to be incorporated at the PSDs. Such dramatic redistribution of receptors had severe consequences on synapse structure and function. In fact, the neto hypomorphic mutant animals are only 50% viable, and the adult escapers cannot fly and have severe locomotor defects. We recorded evoked excitatory junctional potentials (EJPs) and spontaneous miniature potentials (mEJPs, or minis) from muscle 6 of third instar larvae and found a dramatic reduction in mini frequency and amplitude. The amplitude of evoked EJPs was also reduced in the neto hypomorphic mutant animals, but they did not exhibit a presynaptic compensatory response, that is, they did not release more neurotransmitter-filled vesicles to compensate for their reduced postsynaptic sensitivity. Thus, reduced Neto levels significantly impair the number and density of postsynaptic iGluRs without an apparent effect on presynaptic release.
Click image to enlarge.
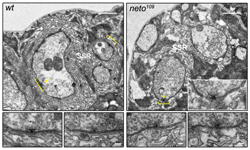
Figure 2. neto mutants exhibit disorganized synapses and no detectable postsynaptic densities.
The active zones are juxtaposed by electron-dense structures (brackets) in the synaptic cleft and postsynaptic domain of wild-type but not Neto-deprived synapses. Bar, 1 μm; in lower panels (details), 0.1 μm.
Finally, Neto-deprived animals exhibit a deficit in the formation and maintenance of mature PSDs. With help from the NICHD Microscopy and Imaging Core Facility, we were able to capture and compare electron micrographs of synaptic boutons from wild-type and Neto-deprived third instar larvae (Figure 2). At wild-type synapses, the active zones (T bars) are juxtaposed to electron-dense structures in the synaptic cleft and postsynaptic domain (brackets). In contrast, the neto109 mutant synapses display a loss of electron-dense membrane domains. The pre- and postsynaptic membranes are sinuous and appear disorganized in neto109 mutants, as compared with the tight and close apposition of wild-type synaptic membranes. During synapse formation, iGluRs incorporation into the postsynaptic membrane is critical to enlarge PSDs. By clustering in concert to iGluRs, Neto is essential for functional iGluR complexes and directly controls synapse formation at the Drosophila NMJ.
Our data fit best with a model in which Neto and the iGluRs are engaged in targeting each other to the PSDs via a direct interaction. Neto likely binds to iGluR complexes on the surface of muscle, followed by trafficking to the synaptic junction. Our model is consistent with Neto/iGluRs co-dependence for clustering at the synapse that we described: only components engaged in a productive complex could traffic and be stabilized at the NMJ. An intriguing question is why the requirements for Neto differ dramatically among various organisms. Neto1/Neto2 double-knockout mice have defects in long-term potentiation and learning and memory but are viable. More important, in mice Neto1 and Neto2 are not essential for iGluRs clustering. In contrast, Drosophila neto-null mutants are embryonic lethal, and Neto is an absolute requirement for the clustering of iGluRs. It is tempting to speculate that Neto has attained tissue- or context-specific roles in the modulation of iGluRs. Thus, Netos constitute a family of proteins conserved from flies to humans that influence the function of glutamatergic synapses and have acquired species- and tissue-specific roles during evolution.
Neto does not contain any catalytic domain; instead, it has several extracellular protein-interaction domains (CUB domains), and two alternative intracellular domains rich in putative phosphorylation sites and docking motifs. Therefore, Neto likely functions in iGluRs synaptic targeting and PSDs stabilization by binding to iGluRs and/or other interaction partners. For example, Neto may promote iGluRs aggregation via CUB–mediated self-association and/or extracellular interactions. We are currently focusing on identifying and characterizing extracellular and intracellular Neto interaction partners.
Formation and interpretation of morphogen gradients
Spatially non-uniform distributions of secreted morphogens guide tissue development in a highly reproducible and robust manner. In the early Drosophila embryo, the BMP-type ligand Decapentaplegic (Dpp) is critical for the assignment of identity to all dorsal structures. Dpp is transcribed uniformly throughout the dorsal domain, yet it forms an activity gradient via a cascade of extracellular regulation that restricts Dpp availability laterally while simultaneously amplifying Dpp activity near the dorsal midline. Formation of the Dpp activity gradient requires several secreted modulators. In the Drosophila early embryo, Dpp is bound in a complex containing Short gastrulation (Sog). The complex inhibits binding of Dpp to its receptors in lateral regions and facilitates long-range ligand diffusion, shuttling Dpp from the lateral domain toward the midline. A crucial step that helps create flux is the cleavage of Sog by Tolloid (Tld), a metalloprotease of the BMP-1 family. The net movement of Dpp dorsally is generated by reiterated cycles of complex formation, diffusion, and destruction by Tld. The BMP signaling domains are further refined by positive feedback and additional secreted BMP modulators, such as Crossveinless-2 (Cv-2), which binds to the cell surface and acts over a short range.
The BMP activity gradient in the early Drosophila embryo is very steep, with sharp boundaries, unlike the shallower BMP gradients usually found in vertebrate systems. In flies, Sog plays both positive and negative roles in regulating BMP activity, a phenomenon previously referred to as the "Sog paradox." The negative role comes from blocking access of ligands to receptors; the positive effect comes from Sog's ability to facilitate Dpp diffusion. Although Chordin is thought to be the functional homologue of Sog, it acts only as an inhibitor and cannot promote long-range Dpp signaling when introduced into Drosophila. At the molecular level, the difference between Sog and Chordin is that cleavage of Sog by Tolloid (Tld) requires the BMP ligand as an obligatory co-substrate, while Chordin does not. We proposed that the BMP dependence of Sog destruction makes Sog a more efficient BMP transporter and results in long-range BMP signaling. To test this hypothesis, we first characterized the Sog-processing sites by Tld and derived a consensus cleavage sequence. We modeled the Tld catalytic domain by using the crystal structures available for related enzymes and studied the enzyme-substrate interactions between Tld and either Sog or Chordin. We found that several residues are responsible for rendering Sog (but not Chordin) destruction dependent on BMP binding. Changing corresponding residues indeed altered co-substrate requirements and rendered Sog "independent of BMP for cleavage," a state designated Sog-i. At the sequence level, Sog-i is markedly different from Chordin but resembles Chordin in how it is processed by Tld: Sog-i exhibits significant BMP–independent processing by Tld, which is enhanced in the presence of BMP ligands.
Replacement of endogenous Sog with Sog-i in early Drosophila embryos broadened the spatial domain of active BMP signaling and produced a shallow-sloped concentration gradient, analogous to that observed in some vertebrate embryos. The change ultimately affected cell-fate allocation and tissue size along the D/V axis. Moreover, our comparison of sog-wt with sog-i embryos suggests that BMP–dependent Sog processing reduces embryo-to-embryo variability of patterning. Our results indicate that a "Chordin-like" Sog, i.e., Sog-i, is less able to reliably support patterning of the early Drosophila embryo than Sog. Thus, the acquisition of BMP–dependent Sog processing during evolution facilitated long-range ligand diffusion and formation of the robust morphogen gradients required for early Drosophila patterning.
Publications
- Kim YJ, Bao H, Bonanno L, Zhang B, Serpe M. Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev 2012;26:974-987.
- Peluso CE, Umulis D, Kim YJ, O'Connor MB, Serpe M. Shaping BMP morphogen gradients through enzyme-substrate interactions. Dev Cell 2011;21:375-383.
Collaborators
- Seth S. Blair, PhD, University of Wisconsin, Madison, WI
- Chi-Hon Lee, PhD, Program on Cellular Regulation and Metabolism, NICHD, Bethesda, MD
- Mark Mayer, PhD, Program in Developmental Neuroscience, NICHD, Bethesda, MD
- Michael B. O’Connor, PhD, University of Minnesota, Minneapolis, MN
- David Umulis, PhD, Purdue University, West Lafayette, IN
- Bing Zhang, PhD, University of Oklahoma, Norman, OK
Contact
For more information, email serpemih@mail.nih.gov or visit ucc.nichd.nih.gov.

