You are here: Home > Section on Cellular and Synaptic Physiology
Hippocampal Interneurons and Their Role in the Control of Network Excitability

- Chris J. McBain, PhD, Head, Section on Cellular and Synaptic Physiology
- Kenneth Pelkey, PhD, Staff Scientist
- Ramesh Chittajulla, PhD, Senior Research Fellow
- Gulcan Akgul, PhD, Postdoctoral Fellow
- Elizabeth Barksdale, PhD, Postdoctoral Fellow
- Michael Craig, PhD, Postdoctoral Fellow
- Jason Wester, PhD, Postdoctoral Fellow
- Megan Wyeth, PhD, Postdoctoral Fellow
- Brian Jeffries, BS, Biologist
- Xiaoqing Yuan, MSc, Biologist
- April Johnston, BSc, Graduate Student
- Geoff Vargish, BS, Graduate Student
- Carla Lopez, BS, NIH Academy
- David Collins, BSc, Postbaccalaureate Fellow
Click image to enlarge.
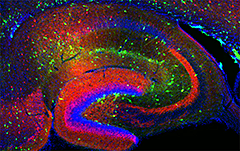
Figure 1. Interneurons in the GAD-65 GFP mouse
Numerous subpopulations are present within a hippocampal section derived from a GAD-65 GFP–transgenic mouse (GAD-65, the enzyme that catalyzes the synthesis of GABA, is a glutamic acid decarboxylase).
Cortical and hippocampal local circuit GABAergic inhibitory interneurons are “tailor-made” to control Na+- and Ca2+-dependent action potential generation, to regulate synaptic transmission and plasticity, and to pace large-scale synchronous oscillatory activity. The axons of this diverse cell population make local, usually short-range projections (some subpopulations project their axons over considerable distances) and release the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) onto a variety of targets. A mounting appreciation of the roles played by interneurons in several mental health conditions such as epilepsy, stroke, Alzheimer's disease, and schizophrenia have placed this important cell type center stage in cortical circuit research. Our main objective is to understand the developmental programs that regulate their integration into cortical circuits and how both ionic and synaptic mechanisms regulate the activity of cortical neurons at the level of small, well defined networks. To this end, we use a variety of electrophysiological, immunohistochemical, molecular, and genetic approaches in both wild-type and transgenic animals. Over the past five years, we have continued our study of the differential mechanisms of glutamatergic and GABAergic synaptic transmission and plasticity within the hippocampal formation and the modulation of voltage- and ligand-gated channels expressed in inhibitory neurons. We also incorporated genetic approaches to unravel the embryogenesis and development of hippocampal interneurons and the circuits in which they are embedded.
Neto auxiliary protein interactions regulate kainate and NMDA–receptor subunit localization at mossy fiber–CA3 pyramidal cell synapses.
Neto1 and Neto2 auxiliary subunits co-assemble with NMDA and kainate receptors (NMDAR and KARs) to modulate their function. In the hippocampus, Neto1 enhances the amplitude and prolongs the kinetics of KAR–mediated currents at mossy fiber–CA3 pyramidal cell (MF–CA3) synapses. However, whether Neto1 trafficks KARs to synapses or simply alters channel properties is unresolved. Therefore, Megan Wyeth and Ken Pelkey used post-embedding electron microscopy to investigate the localization of GluK2/3 subunits at MF–CA3 synapses in Neto-null mice. Postsynaptic GluK2/3 immuno-gold labeling was substantially reduced in Neto-null mice compared with wild type. Moreover, spontaneous KAR–mediated synaptic currents and metabotropic KAR signaling were absent from CA3 pyramidal cells of Neto-null mice. We observed a similar loss of ionotropic and metabotropic KAR function in Neto1, but not Neto2, single knockout mice, specifically implicating Neto1 in regulating CA3 pyramid KAR localization and function. Additional controversy pertains to the role of Neto proteins in modulating synaptic NMDARs. While immuno-gold labeling for GluN2A at MF–CA3 synapses was comparable between wild-type and Neto-null mice, labeling for postsynaptic GluN2B was robustly increased in Neto-null mice. Accordingly, NMDAR–mediated currents at MF–CA3 synapses exhibited elevated sensitivity to a GluN2B–selective antagonist in Neto1 knockouts compared with wild type. Thus, despite preservation of the overall MF–CA3 synaptic NMDAR–mediated current, loss of Neto1 alters NMDAR subunit composition. The results confirm that Neto protein interactions regulate synaptic localization of KAR and NMDAR subunits at MF–CA3 synapses, with implications for both ionotropic and metabotropic glutamatergic recruitment of the CA3 network.
Neurogliaform cells dynamically regulate somatosensory integration via synapse-specific modulation.
The circuitry of layer IV somatosensory (barrel) cortex is exquisitely designed to accurately encode the temporal features of tactile sensory inputs owing to an efficient thalamic recruitment of strong feed-forward inhibition (FFI). FFI, predominantly mediated by parvalbumin (PV)-expressing fast-spiking interneurons (FS INs), imposes a restricted integration time window (IW) for summation of excitatory inputs and confers the temporal precision required for faithful representation of sensory information encoded in thalamic synchrony. Recently, the subtype of IN termed the neurogliaform cell (NGFC) was identified in layer IV of the barrel cortex. NGFCs possess axons with dense arborization containing relatively high numbers of release sites, of which a large proportion are not associated with classical synapses and which result instead in volume transmission of GABA. Despite the potential to interact with large areas of microcircuits in a relatively target-independent manner, very little is known about how NGFC activity influences the function of neural circuits in which they are embedded. We sought to determine the role of NGFCs in the layer IV barrel microcircuit, with a particular focus on their interaction with the canonical thalamic-evoked FFI. Despite the prevailing idea that neurogliaform cells produce a spatially unrestricted widespread inhibition, Ramesh Chittajallu demonstrated that their activity attenuates thalamic-evoked feed-forward inhibition in layer IV barrel cortex but has no effect on feed-forward excitation. The result of this circuit selectivity is dynamic regulation in the temporal window for integration of excitatory thalamic input, thus revealing a novel role for neurogliaform cells in shaping sensory processing.
Dual embryonic origins of functionally distinct hippocampal O-LM cells revealed by differential 5-HT3AR expression
Forebrain circuits rely upon a relatively small but remarkably diverse population of GABAergic interneurons to bind to and entrain large principal-cell assemblies for network synchronization and rhythmogenesis. Despite the high degree of heterogeneity across cortical interneurons, members of a given subtype typically exhibit homogenous developmental origins, neuromodulatory response profiles, morphological characteristics, neurochemical signatures, and electrical features. We found a surprising divergence amongst hippocampal oriens-lacunosum moleculare (O-LM) projecting interneurons that were hitherto considered a homogeneous cell population. Combined immunocytochemical, anatomical, and electrophysiological interrogation of 5-HT3AR-BACGFP and Nkx2-1Cre:RCE mice revealed that O-LM cells parse into caudal ganglionic eminence–derived 5-HT3AR–expressing, and medial ganglionic eminence–derived 5-HT3AR–lacking subpopulations. The two cohorts differentially participate in network oscillations with 5-HT3AR–containing O-LM cell recruitment dictated by serotonergic tone. Thus, members of a seemingly uniform interneuron population can exhibit unique circuit functions and neuromodulatory properties dictated by disparate developmental origins.
Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity.
Disrupted maturation of excitatory synapse in cortical GABAergic interneurons can precipitate an imbalance in circuit excitation/inhibition that is thought to underlie the progression of neuropsychiatric disorders such as schizophrenia. However, establishing the basic developmental programs for nascent synapses in GABAergic cells is confounded by their sparsity, heterogeneity, and the late postnatal acquisition of subtype-defining characteristics. To overcome these hurdles, we investigated the synaptic properties of individual interneurons throughout early postnatal development, targeting cells based on lineage from medial or caudal ganglionic eminence (MGE and CGE) progenitors and thus enabling interrogation of non-overlapping interneuron populations whose fates can be predicted from origin. We found stereotyped developmental differences in synaptic AMPA and NMDA receptors between MGE– and CGE–derived interneurons, including differential GluN2 subunit composition of NMDARs and expression of Ca2+-permeable AMPARs. Our findings establish ganglionic eminence–dependent rules for early postnatal synaptic integration programs of distinct interneuron cohorts including parvalbumin- and cholecystokinin-expressing basket cells.
Additional Funding
- Megan Wyeth was funded by a PRAT Fellowship.
Publications
- Chittajallu R, Craig MT, McFarland A, Yuan X-Q, Gerfen S, Tricoire L, Erkkila B, Barron SC, Lopez CM, Liang BJ, Jeffries BW, Pelkey KA, McBain CJ. Dual embryonic origins of functionally distinct hippocampal O-LM cells revealed by differential 5-HT3AR expression. Nat Neurosci 2013;16:1598-1607.
- Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci 2013;16:1032-1041.
- Mayne EW, Craig MT, McBain CJ, Paulsen O. Dopamine suppresses persistent network activity via D1-like dopamine receptors in rat medial entorhinal cortex. Eur J Neurosci 2013;37:1242-1247.
- Craig MT, Mayne EW, Bettler B, Paulsen O, McBain CJ. Distinct roles of GABAB1a and GABAB1b -containing receptors in spontaneous and evoked termination of persistent cortical activity. J Physiol 2013;591(Pt 4):835-843.
- Chittajallu R, Pelkey KA, McBain CJ. Neurogliaform cells dynamically regulate somatosensory integration via synapse specific modulation. Nat Neurosci 2013;16:13-15.
Collaborators
- Roderick McInnes, PhD, Lady Davis Research Institute, McGill University, Toronto, Canada
- Michael Salter, PhD, Centre for the Study of Pain, Hospital for Sick Children, Toronto, Canada
- Paul Worley, PhD, The Johns Hopkins University, Baltimore, MD
Contact
For more information, email mcbainc@mail.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/chris-mcbain.aspx.

