You are here: Home > Section on Cellular Signaling
Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Melanija Tomić, PhD, Staff Scientist
- Ivana Bjelobaba, PhD, Visiting Fellow
- Marek Kucka, PhD, Visiting Fellow
- Ellias Leiva-Salcedo, PhD, Visiting Fellow
- Milos B. Rokic, PhD, Visiting Fellow
- Paula Bargi de Souza, MS, Special Volunteer
Using multidisciplinary and collaborative approaches, we investigate signaling pathways at cellular and molecular levels, determine the manner in which hormones and neurotransmitters utilize calcium as an intracellular messenger, and characterize channels involved in electrical activity and calcium signaling in hypothalamic, pituitary, and other cell types. We investigated cellular signaling cascades and secretion in neuroendocrine cells, with special emphasis on the interactions between plasma-membrane electrical events and receptor-controlled pathways. Currently, we are determining how the structural features of pituitary channels relate to the channels' functions and how plasma membrane receptors and the intracellular signaling milieu affect channel activity. For this purpose, we characterize native and recombinant channels and receptors that were cloned from the pituitary gland. We also analyze the relevance of these channels and pathways to calcium-dependent cellular processes.
GnRH induction of DMP1 in pituitary gonadotrophs
Hypothalamic gonadotropin-releasing hormone (GnRH) is the primary regulator of reproduction in vertebrates, acting via the G protein–coupled GnRH receptor (GnRHR) in pituitary gonadotrophs to control synthesis and release of gonadotropins. Recently, we sought to increase our understanding of the GnRHR–coupled gene network in non-transformed cells using RNA sequencing of primary pituitary cells from postpubertal female rats treated with GnRH. We applied this gonadotroph-specific agonist in a pulsatile manner for six hours to a mixed population of perifused pituitary cells from cycling females, extracted mRNA, and performed RNA sequencing analysis. This revealed 83 candidate regulated genes, including many coding for secreted proteins. Most notably, GnRH induces a greater than 600-fold increase in expression of the gene encoding dentin matrix protein-1 (Dmp1), one of five members of the small integrin-binding ligand N-linked glycoprotein gene family. The Dmp1 response is mediated by the GnRHR, not elicited by other hypothalamic releasing factors, and is about 20-fold lower in adult male pituitary cells. The sex-dependent Dmp1 response is established during the peripubertal period and independent of the developmental pattern of Gnrhr expression. In vitro, GnRH–induced expression of this gene is coupled with release of DMP1 in extracellular medium through the regulated secretory pathway. In vivo, pituitary Dmp1 expression in identified gonadotrophs is elevated after ovulation. Cell-signaling studies revealed that GnRH induction of Dmp1 is mediated by the protein kinase C signaling pathway and reflects opposing roles of ERK1/2 and p38 MAPK (Figure 1); in addition, the response is facilitated by progesterone. The results establish that DMP1 is a novel secretory protein of female rat gonadotrophs, the synthesis and release of which are controlled by the hypothalamus through the GnRHR signaling pathway. This advance raises intriguing questions about the intrapituitary and downstream effects of this new player in GnRH signaling (1).
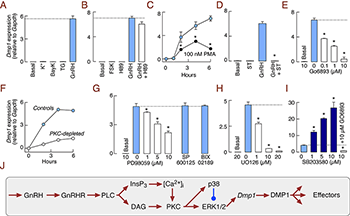
Figure 1. GnRH stimulates Dmp1 expression in the female rat pituitary through the PKC-MAPK signaling pathway.
(A) Elevation of cytosolic calcium concentration by 25 mM KCl (K+), 1 µM BayK 8644 (BayK), or 100 nM thapsigargin (TG) did not elevate Dmp1 expression. (B) Forskolin (FSK; 1 µM), an adenylyl cyclase activator, did not induce Dmp1 expression and H89 (10 µM), an inhibitor of cyclic AMP dependent protein kinase, did not affect basal and GnRH-stimulated Dmp1 expression. (C) PKC activation by phorbol 12-myristate 13-acetate (PMA; 100 nM) elevated Dmp1 expression. (D and E) PKC inhibition by 10 µM staurosporine (D) and Go6983 (E) downregulated GnRH induction of Dmp1. (F) GnRH–stimulated Dmp1 expression was reduced following PKC depletion by treatment with PMA (100 nM, 18 h). (G and H) Inhibition of ERK1/2 with PD98059 (G) or UO126 (H) inhibited GnRH induction of Dmp1; lack of effect of inhibition of JNK and big ERKs with 1 µM 600125 and Bix022189, respectively. (I) p38 MAPK inhibition by SB203580 enhanced GnRH-induced Dmp1 expression; SB203580 enhancement was abolished by Go6983 treatment. Pituitary cells from 7-week-old female rats were treated for 6 h. * Significant differences between PMA and GnRH–stimulated cells (C) and vs. GnRH–stimulated cells (other panels). In all experiments GnRH concentration was 10 nM. (J) Schematic representation of signaling pathways controlling DMP1 expression in pituitary gonadotrophs.
Cholinergic signaling pathways in pituitary gonadotrophs
Acetylcholine (ACh) has been established as a paracrine factor in the pituitary gland, but the receptors mediating ACh action and the cell types bearing the receptors have not been identified. Recently, we completed investigations on the expression and role of these receptors in pituitary gonadotrophs. Our results showed that the expression of the nicotinic subunits' mRNAs followed the order beta2 > beta1 = alpha9 > alpha4 in cultured rat pituitary cells. M4 > M3 muscarinic receptor mRNAs were also identified in pituitary cells. The treatment of cultured pituitary cells with GnRH down-regulated the expression of alpha9 and alpha4 mRNAs, without affecting the expression of M3 and M4 receptor mRNAs, and ACh did not alter the expression of GnRHR mRNA. We also performed double immunostaining to show the expression of beta2 subunit and M4 receptor proteins in gonadotrophs. We identified, in single cells, functional nicotinic channels capable of generating an inward current and facilitating electrical activity and calcium influx. The M3 receptor–mediated, phospholipase C–dependent calcium mobilization activated an outward apamin-sensitive potassium current and caused hyperpolarization (Figure 2). The activation of M4 receptors by ACh inhibited cAMP production and GnRH–induced luteinizing hormone (LH) release. We concluded that multiple cholinergic receptors are expressed in gonadotrophs and that the main secretory action of ACh is inhibitory through M4 receptor–mediated down-regulation of cAMP production. The expression of nicotinic receptors in vitro compensates for the lack of regular GnRH stimulation of gonadotrophs (2).
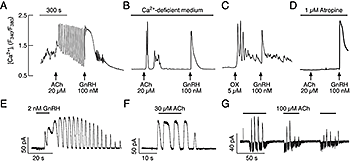
Figure 2. Agonist-induced oscillatory calcium and current responses in identified gonadotrophs
(A–B) Examples of calcium responses to application of ACh and GnRH in gonadotrophs bathed in calcium-containing (A) and calcium-deficient (B) medium. (C–D) Oxotremorine (OX) stimulated calcium oscillations (C) and atropine inhibited effects of ACh on calcium response (D) in GnRH–responsive cells bathed in calcium-deficient medium. In this and following figures, arrows indicate the start of agonist application. (E–F) Voltage clamp amphotericin-perforated whole-cell recording of calcium oscillations monitored as an outward calcium-activated current in gonadotrophs stimulated with GnRH (E) and ACh (F). (G) In a fraction of gonadotrophs, ACh stimulated inward and outward currents. All cells were voltage-clamped at –60 mV. Horizontal bars indicate duration of agonist application.
Cyclic nucleotide signaling in pituitary cells
The cyclic nucleotides cAMP and cGMP are intracellular messengers that are produced from the nucleotide triphosphates by a family of enzymes consisting of adenylyl and guanylyl cyclases. Our previous work in pituitary cells revealed that the cyclic nucleotides and their kinases play important roles in the control of electrical activity of the endocrine pituitary cells and channels involved in this process. More recently, we focused on the role of these messengers in the control of hormone secretion downstream of electrical activity in calcium signaling. The hormone release studies show that prolactin (PRL) release from isolated rat lactotrophs stimulated by forskolin, an activator of adenylyl cyclases, and dbcAMP, the membrane-permeable cAMP analog, exhibit a biphasic concentration dependency. Although at lower concentrations (2–10 µM forskolin and 2.5–5 mM dbcAMP), the agents stimulate PRL release, an inhibition is measured at higher concentrations. We recorded discrete increases in high-resolution capacitance, which represent elementary exocytic events. Elevation of cAMP leaves the frequency of full-fusion events unchanged but increases the frequency of transient events, which exhibited a wider fusion pore, as measured by increased fusion-pore conductance and a prolonged fusion-pore dwell-time. The probability of observing rhythmic reopening of transient fusion pores was elevated by dbcAMP. In conclusion, cAMP–mediated stabilization of wide fusion pores prevents vesicles from proceeding to the full-fusion stage of exocytosis, which hinders vesicle content discharge at high cAMP concentrations (3).
Many endocrine disrupters affect cyclic nucleotide signaling in target cells. Atrazine, one of the most commonly used herbicides worldwide, acts as an endocrine disruptor, but the mechanism of its action has not been characterized. In a recent study, we showed that atrazine rapidly increases cAMP levels in cultured rat pituitary and testicular Leydig cells, but less effectively than 3-isobutyl-1-methylxanthine, a competitive non-specific inhibitor of phosphodiesterases (PDEs). In cells treated with forskolin and probenecid (an inhibitor of cyclic nucleotide transporters), but not with 3-isobutyl-1-methylxanthine, atrazine further increased cAMP levels, indicating that inhibition of PDEs accounts for accumulation of cAMP. In contrast to cAMP, atrazine did not alter cGMP levels, further indicating that it inhibits cAMP–specific PDEs. Atrazine-induced changes in cAMP levels were sufficient to stimulate PRL release in pituitary cells and androgen production in Leydig cells, indicating that atrazine acts as an endocrine disrupter both in cells that secrete by exocytosis of prestored hormones and in cells that secrete by de novo hormone synthesis. Rolipram abolished the stimulatory effect of atrazine on cAMP release in both cell types, suggesting that it acts as an inhibitor of PDE4s, isoforms whose mRNA transcripts dominate in these cells together with mRNA for PDE8A. The results indicate that atrazine acts as a general endocrine disrupter by inhibiting cAMP–specific PDE4s.
Gating properties of pituitary purinergic receptor channels
The ATP–gated P2X7 receptor (P2X7R) is natively expressed in pituitary cells, operates as a cytolytic and apoptotic receptor, but also controls sustained cellular responses, including cell growth and proliferation. However, it has not been clarified how the same receptor mediates such opposing effects. To address this question, we combined electrophysiological, imaging, and mathematical studies, using wild-type and mutant rat P2X7Rs. Activation of naive receptors by low agonist concentrations caused monophasic slow desensitizing currents and internalization of receptors without other changes in the cellular morphology, much like other P2XRs. In contrast, saturating agonist concentrations induced high-amplitude biphasic currents, reflecting pore dilation and causing rapid cell swelling and lysis. The existence of the two signaling patterns was accounted for using a revised Markov state model that included, in addition to naive and sensitized states, desensitized states. Occupancy of one or two ATP–binding sites of naive receptors favored a slow transition to desensitized states, whereas the occupancy of the third binding site favored a transition to sensitized/dilated states. Consistent with model predictions, non-dilating P2X7R mutants always generated desensitizing currents. The results suggest that the level of saturation of the ligand-binding sites determines the nature of the P2X7R gating and cellular actions (4).
We also made progress in our investigation of the gating properties of P2X4R, which is natively expressed in pituitary lactotrophs. In general, activation of P2XR causes an enlargement of the receptor extracellular vestibule, leading to opening of the transmembrane pore, but specific roles of vestibule amino acid residues in receptor activation have not been evaluated systematically. Our alanine or cysteine scanning mutagenesis of V47-V61 and F324-N338 sequences of the rat P2X4R expressed in HEK293 cells revealed that V49, Y54, Q55, F324, and G325 mutants were poorly responsive to ATP. The Y54F and Y54W mutations, but not the Y54L mutation, rescued receptor function, suggesting that an aromatic residue is important at this position. The Y54A and Y54C receptor function was partially rescued by ivermectin, suggesting reduced potency of ATP to activate P2X4R. The Q55T, Q55N, Q55E, and Q55K mutations resulted in non-responsive receptors, and only the Q55E mutant was ivermectin-sensitive. The F324L, F324Y, and F324W mutations also rescued receptor function, ivermectin action on channel gating was preserved in all mutants, and changes in ATP responsiveness correlated with the hydrophobicity and side chain volume of the substituent. The G325P mutant had a normal response to ATP, suggesting that G325 is a flexible hinge. A topological analysis revealed that the G325 and F324 residues disrupt a βeta-sheet upon ATP binding. The results point to multiple roles of the extracellular vestibule amino acid residues in the P2X4R function: the V49 residue is important for receptor trafficking, the Y54 and Q55 residues play a critical role in channel gating, and the F324 and G325 residues cause vestibule widening (5).
Publications
- Kucka M, Bjelobaba I, Clokie SJH, Klein DC, Stojilkovic SS. Female-specific induction of rat pituitary dentin matrix protein-1 by GnRH. Mol Endocrinol 2013;27:1840-1855.
- Zemkova H, Kucka M, Bjelobaba I, Tomic M, Stojilkovic SS. Multiple cholinergic signaling pathways in pituitary gonadotrophs. Endocrinology 2013;154:421-433.
- Calejo AI, Jorgacevski J, Kucka M, Kreft M, Goncalves PP, Stojilkovic SS, Zorec R. cAMP-mediated stabilization of fusion pores in cultured rat pituitary lactotrophs. J Neurosci 2013;33:8068-8078.
- Khadra A, Tomic M, Yan Z, Zemkova H, Sherman A, Stojilkovic SS. Dual gating mechanism and function of P2X7 receptor channels. Biophys J 2013;104:2612-2621.
- Rokic MB, Stojilkovic SS, Varva V, Kuzyk P, Tvrdonova V, Zemkova H. Multiple roles of the extracellular vestibule amino acid residues in the function of the rat P2X4 receptor. Plos One 2013;8:e59411.
Collaborators
- Anmar Khadra, PhD, McGill University, Montreal, Canada
- David C. Klein, PhD, Program on Developmental Endocrinology and Genetics, NICHD, Bethesda
- Radmila Kovacevic, PhD, University of Novi Sad, Novi Sad, Serbia
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Hana Zemkova, PhD, Institute of Physiology, Czech Academy of Sciences, Prague, Czech Republic
- Robert Zorec, PhD, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Contact
For more information, email stankos@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx.