You are here: Home > Unit on Cellular Communication
Mechanisms of Cellular Communication During Development

- Mihaela Serpe, PhD, Head, Unit on Cellular Communication
- Young-Jun Kim, PhD, Visiting Fellow
- Cathy Ramos, PhD, Visiting Fellow
- Qi Wang, PhD, Visiting Fellow
- Mikolaj Sulkowski, PhD, Postdoctoral Fellow
- Oghomwen Igiesuorobo, BS, Postbaccalaureate Fellow
- Peter Nguyen, Biological Laboratory Technician
The purpose of our research is to elucidate molecular mechanisms that regulate cell-cell communication during development. We are specifically interested in two related questions in cellular communication: i) how tissues are patterned and correctly connected by long-range signals, and ii) how cells' structures and functions are coordinated at short range with those of their neighbors. To address these questions, we focus on early developmental patterning and on development of a specialized cell-cell interaction zone, the neuromuscular junction (NMJ). Bone morphogenetic proteins (BMPs) accomplish diverse patterning via long-range and short-range signaling. BMPs are also utilized to modulate growth, development and homeostasis at the Drosophila NMJ, a glutamatergic synapse similar in structure and function to vertebrate central synapses. In flies, each NMJ is unique and identifiable, synapses are large and accessible for electrophysiological and optical analysis, making the Drosophila NMJ a favorite genetic system to study synapse development. The subunits that form the glutamate-gated ion channels (iGluRs) are known and relatively well studied. However, the mechanisms that control iGluR clustering and stabilization at the postsynaptic densities (PSDs) remain a mystery. We recently discovered a novel protein, Neto (Neuropillin and Tolloid-like), that is essential for functional receptors. Our findings provide an entry point to understand the molecular mechanisms of synapse development.
Drosophila NMJ is a well established model to study synapse development.
L-glutamate is the primary neurotransmitter at excitatory synapses in the vertebrate CNS and at arthropod neuromuscular junctions (NMJs). However, the molecular mechanisms that trigger the recruitment of glutamate receptors and promote their stabilization at postsynaptic densities remain poorly understood.
In flies as in vertebrates, before a muscle is innervated, low levels of postsynaptic neurotransmitter receptors are present diffusely in the muscle membrane. Once the motor-neuron growth cone arrives at its target muscle, the postsynaptic receptors begin to concentrate at the synaptic cleft. The mechanisms by which neuronal arrival regulates clustering of postsynaptic nicotinic acetylcholine receptors at the vertebrate NMJ are relatively well understood. Clustering and stabilization of glutamate receptors at postsynaptic locations remain the focus of intense research. Live studies at the Drosophila NMJ demonstrated that glutamate receptor clusters are immobilized at post-synaptic densities (PSDs) in large, stable aggregates that could grow up to a clearly defined maximum size. The NMJ grows by adding new synaptic contacts and by enlarging the existing synapses to maximum size. During development, several trans-synaptic signals coordinate the growth of synaptic structures. At the Drosophila NMJ, the signals include an anterograde Wnt signaling pathway and a retrograde BMP signaling pathway. Furthermore, a retrograde signal ensures constant excitability/synapse homeostasis by increasing the presynaptic release of neurotransmitter-filled vesicles in response to diminished postsynaptic sensitivity. Although the nature of this retrograde signal remains to be determined, the homeostatic response can only be triggered when the BMP–signaling pathway components are in place.
We focus on several key questions in neurobiology: i) how iGluRs traffic to the synapse; ii) how they form stable clusters at the mature PSDs; iii) the nature of the neuronal cue that triggers iGluRs clustering at the onset of synaptogenesis; iv) how cellular communication balances synapse activity with synapse growth and maturation.
Drosophila Neto is an essential auxiliary subunit required for functional channels.
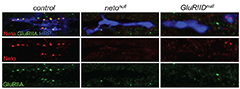
Click image to enlarge.
Figure 1. Neto and iGluRs depend on each other for trafficking and clustering at Drosophila NMJ.
Neto (red) and GluRIIA (green) form clusters at nascent synapses in embryos 21 h after egg laying. The anti-HRP antibody (blue) labels all motor neuron arbors. The GluRIIA receptors do not cluster in the absence of Neto, and Neto does not cluster in the absence of iGluRs.
We recently discovered a novel molecule, Neto (Neurolillin and Tolloid-like), that is absolutely required for clustering and stabilization iGluRs at the Drosophila NMJ (4). Neto belongs to a family of highly conserved proteins sharing an ancestral role in formation and modulation of glutamatergic synapses. While neto genes are not essential in vertebrates or C. elegans nor Neto proteins required for functional receptors, Drosophila Neto is absolutely required for functional iGluRs. We found that Drosophila Neto associates with iGluRs in vivo and controls their trafficking and stabilization at NMJ synapses. The iGluRs of the Drosophila NMJ are hetero-tetrameric complexes composed of three essential subunits—GluRIIC, GluRIID, and GluRIIE—and either GluRIIA or GluRIIB. Embryos lacking any of the essential subunits, or GluRIIA and GluRIIB together, are paralyzed and cannot hatch into the larval stages. Likewise, Drosophila neto mutants are homozygous lethal; they die as paralyzed embryos and lack any body wall peristalsis or hatching movements. In normal animals, Neto concentrates at the NMJ and co-localizes with the iGluRs at postsynaptic densities in puncta juxtaposing the active zones. The essential iGluR subunits are required not only for viability but also for the clustering of the other iGluRs at the NMJ. In the absence of any essential subunit, none of the iGluRs cluster at the NMJ. Intriguingly, iGluRs do not cluster at neto null mutant NMJs, and conversely Neto does not form synaptic clusters in the absence of iGluRs (Figure 1). Thus, Neto and iGluRs depend on each other for trafficking and clustering at synaptic locations. The similarities in subcellular localization and phenotypes between Neto and iGluRs indicate that Neto functions as a non-channel subunit of the iGluRs complexes. By clustering to iGluRs, Neto is essential for functional iGluR complexes and directly controls synapse formation at the Drosophila NMJ.
Neto limits iGluRs trafficking and stabilization at synaptic locations.
Given that Neto is critical for iGluRs trafficking and clustering at synaptic location, perturbations in Neto levels are expected to alter the iGluRs distribution in the striated muscle. We tested this hypothesis by genetically manipulating the levels of Neto in striated muscles and examining the NMJ morphology and physiology. Animals with reduced Neto levels (as in hypomorphic alleles or in RNAi knockdowns) had severe defects in postsynaptic localization of iGluRs. Variations in Neto levels did not affect the net levels of receptors in the muscles, as determined by Western blots, but the immunoreactivity of iGluRs was shifted from postsynaptic to extrajunctional locations. Such a dramatic redistribution of receptors had severe consequences on synapse structure and function. First, Neto-deprived animals exhibited a deficit in the formation and maintenance of mature PSDs. Histological and electron microscopy studies performed at the NICHD Microscopy and Imaging Core Facility indicated that Neto-deprived NMJs exhibit severely altered postsynaptic differentiation and virtually no PSD structures. Secondly, Neto-deprived NMJs show reduced synaptic excitability, with much diminished mini frequency and amplitude and reduced evoked excitatory junctional potentials (EJPs). Interestingly, Neto-deprived NMJs do not exhibit a presynaptic compensatory response, that is, they do not release more neurotransmitter-filled vesicles to compensate for their reduced postsynaptic sensitivity. Lastly, Neto-deprived NMJs are smaller with reduced numbers of boutons and synaptic contacts, suggesting that Neto may influence one of the several signaling pathways known to modulate the NMJ development.
Interestingly, neto overexpression in the muscle also induced abnormal synapse development. NMJs with excess Neto are smaller in size and highly ramified, but their postsynaptic structures are normal. Excess Neto accumulates, in a dose-dependent manner, at NMJ synapses and at extrajunctional locations and appears to sequester iGluRs away from synapses. Similar to Neto-deprived synapses, synapses with excess Neto levels exhibit reduced evoked potentials, without any compensatory increase in quantal contents. Lack of homeostatic compensatory response at Neto-deprived synapses is reminiscent of severely compromised iGluR–deprived synapses. How excess Neto uncouples quantal size reduction from the compensatory increase in presynaptic vesicle release remains a mystery. Neto may sense the status of synaptic iGluRs and relay it to the homeostasis apparatus. Excess Neto may signal “plenty” of postsynaptic iGluRs and consequently override this response.
Furthermore, Drosophila Neto contains an inhibitory prodomain that must be removed by Furin1-mediated cleavage to enable Neto activities. Unprocessed Neto retains its normal subcellular localization and ability to engage iGluRs in vivo, but cannot sustain stable incorporation of iGluRs at PSDs and proper postsynaptic differentiation.
Synaptic pMad as a molecular sensor of synapse activity
Besides its role in receptor clustering and formation and maintenance of PSDs, Neto appears to have additional functions during synapse development. Suboptimal Neto levels affect synapse morphology and physiology, but also synapse size. The reduced growth of Neto-deprived synapses is reminiscent of defects in the BMP retrograde signaling. At the Drosophila NMJ, Glass bottom boat (Gbb), a BMP-type ligand secreted by the muscle, provides a retrograde signal that promotes synaptic growth and confers competency for synaptic homeostasis. Similar to neto, disruptions of BMP signaling pathway components result in small synapses with diminished activity.
Retrograde BMP signaling triggers accumulation of phosphorylated Smad (pMad), a pathway effector, in motor-neuron nuclei and at synaptic termini. In conjunction with other transcription factors, nuclear pMad modulates expression of BMP target genes and promotes synaptic growth. The function of synaptic pMad remains less understood. Given that BMP signals are generally short-lived, synaptic pMad likely reflects accumulation of active BMP/BMP receptor complexes at synaptic termini.
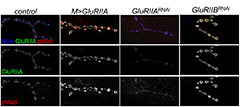
Click image to enlarge.
Figure 2: Synaptic pMad correlates with the postsynaptic type-A glutamate receptors.
Confocal images of third instar NMJs stained with anti-GluRIIA antibodies (green), anti-Neto (blue), and anti-pMad (red). Overexpression of GluRIIA produces an increase in pMad signal intensities compared with controls. Reducing the GluRIIA levels leads to reduction of pMad signals, but reducing the GluRIIB levels has the opposite effect.
We found that pMad signals are selectively lost at NMJ synapses with reduced postsynaptic sensitivities. Despite the loss of synaptic pMad, nuclear pMad persists in motor neuron nuclei, and expression of BMP target genes is unaffected, indicating a specific impairment in pMad production/maintenance at synaptic termini. During development, synaptic pMad accumulation follows the arrival and clustering of iGluRs at NMJ synapses. Excess Neto did not affect the intensity and appearance of synaptic pMad signals, which remained organized in distinct puncta, raising the possibility that synaptic pMad correlates with clustered Neto/iGluRs.
Indeed genetic manipulations of postsynaptic iGluRs or Neto revealed that synaptic pMad is reduced at diminished receptor levels and specifically correlates with type-A channels at PSDs (Figure 2). At the Drosophila NMJ, GluRIIA and GluRIIB subunits compete with each other for the limiting essential subunits GluRIIC, GluRIID, and GluRIIE. The type-A receptors are determinants of quantal size, the postsynaptic response to the spontaneous fusion of a single synaptic vesicle. Furthermore, synaptic pMad appears to correlate with the activity and not synaptic levels of type-A receptors: synaptic pMad signals are diminished by muscle expression of activated protein kinase A, which reduces quantal size without affecting the net levels of synaptic receptors. While future experiments will be needed to address the nature and function of local pMad–containing complexes, our findings clearly demonstrate that synaptic pMad constitutes an exquisite monitor of synapse activity status and thus has the potential to relay information about synapse activity to both pre- and postsynaptic compartments and contribute to synaptic plasticity. Given that BMP signaling plays a crucial role in synaptic growth and homeostasis at the Drosophila NMJ, the use of synaptic pMad as a sensor for synapse activity may enable the BMP signaling pathway to monitor synapse activity and then function to adjust synaptic growth and stability during development and homeostasis.
Publications
- Sulkowski M, Kim YJ, Serpe M. Postsynaptic glutamate receptors regulate local BMP signaling at the Drosophila neuromuscular junction. Development 2013;in press.
- Wharton K, Serpe M. Fine-tuned shuttles for bone morphogenetic proteins. Curr Opin Genet Dev 2013;23:374-384.
- Kim YJ, Serpe M. Making a synapse: a complex matter. Fly 2013;146-152.
- Kim YJ, Bao H, Bonanno L, Zhang B, Serpe M. Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev 2012;26:974-987.
Collaborators
- Seth S. Blair, PhD, University of Wisconsin, Madison, WI
- Chi-Hon Lee, MD, PhD, Program in Cellular Regulation and Metabolism, NICHD, Bethesda, MD
- Mark Mayer, PhD, Program in Developmental Neuroscience, NICHD, Bethesda, MD
- Bing Zhang, PhD, University of Oklahoma, Norman, OK
Contact
For more information, email serpemih@mail.nih.gov or visit ucc.nichd.nih.gov.

