You are here: Home > Section on Molecular Morphogenesis
Thyroid Hormone Regulation of Vertebrate Postembryonic Development

- Yun-Bo Shi, PhD, Head, Section on Molecular Morphogenesis
- Liezhen Fu, PhD, Staff Scientist
- Thomas Miller, PhD, Postdoctoral Intramural Research Training Award Fellow
- Morihiro Okada, PhD, Visiting Fellow
- Julia Rodiger, PhD, Visiting Fellow
- Luan Wen, PhD, Visiting Fellow
- Yu Zhang, PhD, Visiting Fellow
- Nga Luu, MS, Biologist
This laboratory investigates the molecular mechanisms of thyroid hormone (TH) function during postembryonic development. The principal models used are the metamorphosis of Xenopus laevis and X. tropicalis, two highly related species that offer unique but complementary advantages. The control of this developmental process by TH offers a paradigm to study gene function in postembryonic organ development. During metamorphosis, various organs undergo vastly different changes. Some, like the tail, undergo complete resorption, while others, such as the limb, are developed de novo. The majority of larval organs persist through metamorphosis but are dramatically remodeled to function in a frog. For example, tadpole intestine, a simple tubular structure consisting primarily of a single layer of larval epithelial cells, is transformed during metamorphosis into an organ with a multiply folded adult epithelium surrounded by elaborate connective tissue and muscles through specific larval epithelial cell death and de novo development of the adult epithelial stem cells, followed by their proliferation and differentiation. The wealth of knowledge from past research and the ability to manipulate amphibian metamorphosis both in vivo, by using genetic approaches or hormone treatment of whole animals, and in vitro in organ cultures, offer an excellent opportunity to (i) study the developmental function of TH receptors (TRs) and the underlying mechanisms in vivo and (ii) identify and functionally characterize genes that are critical for postembryonic organ development in vertebrates. Our studies have revealed likely conserved mechanisms in adult intestinal stem cell development in vertebrates, which has prompted us to adapt the mouse model to complement the amphibian system for gene knockout studies.
Cytological and morphological analyses reveal distinct features of intestinal development during Xenopus tropicalis metamorphosis.
We have been using intestinal remodeling in Xenopus laevis as a model to study how TH regulates metamorphosis via gene regulation by the heterodimers between TR and RXR (9-cis retinoic acid receptor). Recent advances in genome sequencing and genetic tools for functional studies in vivo have made Xenopus tropicalis a superior model for many studies. To establish X. tropicalis intestinal metamorphosis as a model for adult organogenesis, we analyzed the morphological and cytological changes in X. tropicalis intestine during metamorphosis (1). We observed that, in X. tropicalis, the premetamorphic intestine is made mainly of a monolayer of larval epithelial cells surrounded by sparse connective tissue except in the single epithelial fold, the typhlosole. During metamorphosis, the larval epithelium degenerates, and adult epithelium develops to form a multi-folded structure with elaborate connective tissue and muscles. Interestingly, the typhlosole, which is critical for adult epithelial development, is present along the entire length of the small intestine in premetamorphic X. tropicalis tadpoles, in contrast to X. laevis, where it is present only in the anterior third. TH treatment induces intestinal remodeling, including shortening of the intestine and the typhlosole, just like in X. laevis. Our observations indicate that the intestine undergoes similar metamorphic changes the two related species, making it possible to use the large amount of molecular information available on X. laevis intestinal metamorphosis and the genome sequence information and genetic advantages of X. tropicalis to dissect the pathways governing adult intestinal development.
Genome-wide analysis identified direct target genes of TR during intestinal remodeling.
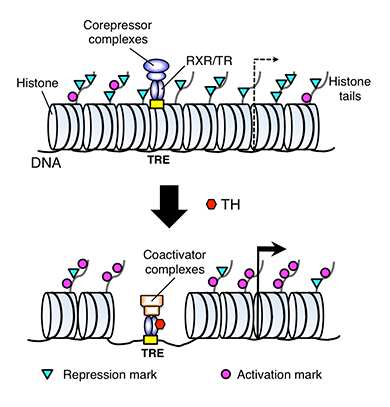
Figure 1. A model for gene regulation by TR
TH functions by regulating gene transcription through TH receptors (TRs). In the absence of TH (as in the premetamorphic tadpole), TR forms heterodimers with RXR (9-cis retinoic acid receptor), and the heterodimer binds to the TH response elements (TREs) in the target genes to repress the latter's expression by recruiting corepressor complexes containing histone deacetylases. When TH is present, the corepressor complexes are released, and liganded TR/RXR recruits coactivator complexes containing histone acetyltransferases and histone methyltransferases such as PRMT1 (protein arginine methyltransferase 1). The coactivator complexes modify histones or cause the removal of nucleosomes, leading to the activation of gene expression.
TH functions by regulating gene transcription through TRs. TRs are transcription factors that bind directly to target genes to activate or repress transcription in a TH-dependent manner, a process that involves histone modifications and chromatin remodeling (Figure 1). In TH-induced metamorphosis, the direct target genes of TR are the participants immediately downstream of TH signal. To identify such genes, we carried out a chromatin-immunoprecipitation (ChIP)-on-chip study on the intestine of premetamorphic X. tropicalis tadpoles treated with or without TH by using a set of microarray chips covering a 8 kb region flanking each putative promoter of 17,000 Xenopus tropicalis genes to look for genes bound by TR. Preliminary analyses of the ChIP-on-chip data led to the identification of many putative TR target genes. Among them are the genes encoding iodotyrosine deiodinase (IYD) and Dot1-Like (Dot1L). Dot1L is the homolog of the yeast Dot1 gene, originally identified as a disruptor of telomeric silencing in Saccharomyces cerevisiae, and belongs to the family of lysine methyltransferases. To date, Dot1L is the only known methyltransferases that possesses histone methyltransferase activity toward histone H3 lysine (K) 79. IYD is believed to be involved in the recycling of iodine from the byproducts of TH biosynthesis and metabolism: 3′-monoiodotyrosine and 3′, 5′-diiodotyrosine. Our further analyses of these two genes not only confirmed the binding of TR to their promoter regions in vivo but also revealed the binding sites responsible for their strong induction by TH (2). Furthermore, expression studies supported their likely role in intestinal metamorphosis. Thus, it is quite likely the other candidate genes identified from the ChIP-on-chip assay are also true target genes of TR and participate in metamorphosis as well.
Expression profiling of intestinal tissues implicates tissue-specific genes and pathways essential for thyroid hormone–induced adult stem cell development.
We had shown earlier that the de novo formation of adult intestinal stem cells requires TH signaling in both the larval epithelium (Ep) and non-epithelial tissues (non-Ep). To understand the underlying molecular mechanisms, it is critical to determine the genes and signaling pathways involved in this process. Thus, we profiled the gene expression programs in epithelial and non-epithelial tissues in X. laevis intestine to systematically determine changes underlying the cell-autonomous and cell-cell interaction–dependent processes that are required for stem cell formation. We isolated the intestines of tadpoles from pre-metamorphosis (stage 56), metamorphic climax (stage 61/62, when larval epithelial cell death is near completion, cell proliferation is rapid, and most of the cells are adult progenitor/stem cells), and end of metamorphosis (stage 66, when adult intestine is formed) and separated the Ep from the non-Ep of each intestine. High-throughput gene expression profiling on the isolated tissues revealed that most genes regulated during intestinal metamorphosis are tissue-specific and that distinct signal transduction pathways are affected by T3 in the Ep and non-Ep, suggesting differential roles for these genes/signal transduction pathways in cell autonomous functions in stem cell development vs. in the stem cell niche formation (3). Our results further suggest the potential existence of many novel stem cell–associated genes, at least in the developing intestine.
Tissue-specific upregulation of MDS/EVI gene transcripts in the intestine by thyroid hormone during Xenopus metamorphosis
One of the genes that we discovered to be highly expressed in the epithelium at the climax of metamorphosis, when most of the epithelial cells are adult progenitor/stem cells, is the homolog of human ectopic viral integration site 1 (EVI). The EVI locus encodes EVI as well as its variant myelodysplastic syndrome 1 (MDS)/EVI, which is generated via transcription from the upstream MDS promoter and alternative splicing. EVI and MDS/EVI have been implicated in several cancers, including breast, leukemia, ovarian, and intestinal cancers. We showed that EVI and MDS/EVI transcripts were upregulated by TH in the epithelium but not in the rest of the intestine in Xenopus laevis when adult stem cells are forming in the epithelium (4). Our results suggest that EVI and MDS/EVI are likely involved in the development and/or proliferation of newly forming adult intestinal epithelial cells.
Genetic analysis of the function of a thyroid hormone/amino acid transporter during mouse development
To regulate cellular processes, TH must be actively transported into cells, a process that is mediated by several different types of transporters. LAT1, one of our previously identified TH-response genes in the intestine, encodes the light chain of a heterodimeric system L type of TH transporter, which also transports several amino acids. Interestingly, LAT1 is highly upregulated at the climax of metamorphosis in the tadpole intestine, coinciding with the formation and rapid proliferation of the adult intestinal stem cells. We recently found that LAT1 is also highly expressed in the mouse intestine during the neonatal period, when the mouse intestine matures into the adult form, a process that appears to also involve TH-dependent formation and/proliferation of adult intestinal stem cells. In a collaborative study, we generated a mouse line with the LAT1 gene floxed, which allows conditional knockout of LAT1 upon expression of the Cre recombinase. While we are still in the process of investigating whether LAT1 affects adult intestinal stem cell development in the mouse, we recently showed, in another collaboration, that LAT1 is critical for antigen receptor–mediated metabolic re-programming (5), a process essential for T cell differentiation.
Publications
- Sterling J, Fu L, Matsuura K, Shi Y-B. Cytological and morphological analyses reveal distinct features of intestinal development during Xenopus tropicalis metamorphosis. PLoS One 2012;7, e47407:1-10.
- Fujimoto K, Matsuura K, Das B, Fu L, Shi Y-B. Direct activation of Xenopus iodotyrosine deiodinase by thyroid hormone receptor in the remodeling intestine during amphibian metamorphosis. Endocrinology 2012;153:5082-5089.
- Sun G, Heimeier RA, Fu L, Hasebe T, Das B, Ishizuya-Oka A, Shi Y-B. Expression profiling of intestinal tissues implicates tissue-specific genes and pathways essential for thyroid hormone-induced adult stem cell development. Endocrinology 2013;Epub ahead of print.
- Miller TC, Sun G, Hasebe T, Fu L, Heimeier RA, Das B, Shi Y-B. Tissue-specific upregulation of MDS/EVI gene transcripts in the intestine by thyroid hormone during Xenopus metamorphosis. PLoS One 2013;8, e55585:1-7.
- Sinclair LV, Rolf J, Emslie E, Shi Y-B, Taylor PM, Cantrell DA. Antigen receptor control of amino acid transport coordinates the metabolic re-programming that is essential for T cell differentiation. Nat Immunol 2013;14:500-508.
Collaborators
- Michael Bustin, PhD, Center for Cancer Research, NCI, Bethesda, MD
- Doreen A. Cantrell, PhD, University of Dundee, Dundee, UK
- Chuxia Deng, PhD, Genetics of Development and Disease Branch, NIDDK, Bethesda, MD
- Atsuko Ishizuya-Oka, PhD, Nippon Medical School, Tokyo, Japan
- Peter Taylor, PhD, University of Dundee, Dundee, UK
Contact
For more information, email shi@helix.nih.gov or visit smm.nichd.nih.gov.

