Regulation of Childhood Growth
- Jeffrey Baron,
MD, Chief, Section on Growth and Development - Lesley Brown, PhD, Staff Scientist
- Julian Lui, PhD, Staff Scientist
- Krishma Tailor, PhD, Biologist
- Wei Wang, MSc, Biologist
- Kirtal Hansdah, PhD, Visiting Fellow
- Fatima Elzamzami, BS, Postbaccalaureate Fellow
- Isabelle Hannula, BS, Postbaccalaureate Fellow
- Arun Rama-Krishnan, BS, Postbaccalaureate Fellow
- Connor Sisk, BS, Postbaccalaureate Fellow

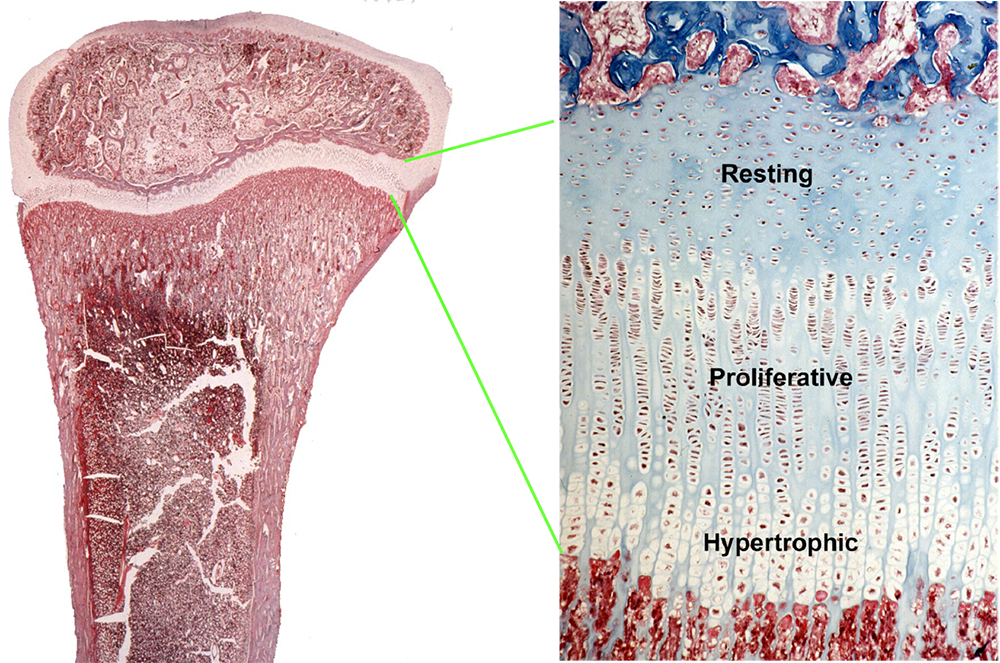
Children grow taller because their bones grow longer. Bone elongation occurs at the growth plate, a thin layer of cartilage found near the ends of juvenile bones (Figure 1). In the growth plates, new cartilage is produced through chondrocyte proliferation, hypertrophy, and cartilage matrix synthesis, and then the newly formed cartilage is remodeled into bone. The process, termed endochondral ossification, results in bone elongation, which causes children to grow in height.
We investigate the cellular and molecular mechanisms governing childhood growth and development. We focus particularly on growth at the growth plate, which drives bone elongation. One goal of this work is to gain insight into the many human genetic disorders that cause childhood growth failure or overgrowth. A second goal is to develop new treatments for children with severe growth disorders.
Novel genetic causes of childhood growth disorders
Variants in genes that affect the growth plate often impair chondrogenesis, causing short stature. More severe genetic abnormalities cause bones to be malformed, presenting clinically as a skeletal dysplasia. If the genetic defect affects tissues other than the growth-plate cartilage, the child may present with a more complex syndrome that includes other clinical abnormalities. Less commonly, mutations in these genes cause excessive growth-plate chondrogenesis and therefore abnormally tall stature. Often the increased proliferation occurs in many tissues, producing a generalized overgrowth syndrome, which can include other medical problems such as developmental delay and increased cancer risk.
For many children with growth disorders, clinical, laboratory, and genetic evaluation fails to identify the underlying etiology. To discover new genetic causes of childhood growth disorders, we evaluated families with monogenic growth disorders using exome sequencing. The identified sequence variants and the genes in which they occur were studied in the laboratory to confirm that the variant is pathogenic, to elucidate the pathogenesis of the disorder, and to explore the role of the gene in normal growth.
With this approach, our group identified multiple new causes of childhood growth disorders. We discovered that variants in aggrecan (ACAN), a component of cartilage extracellular matrix, cause autosomal-dominant short stature with advanced skeletal maturation, and that such patients tend to develop early-onset osteoarthritis. We also found that variants in QRICH1, which positively regulates the transcription of genes that regulate protein secretion, cause a chondrodysplasia resulting from impaired growth-plate chondrocyte hypertrophic differentiation. We also found evidence that heterozygous deletion of CYP26A1 and CYP26C1, which encode enzymes that metabolize retinoic acid, accelerate bone and dental maturation in humans and cause developmental defects involving the eye and central nervous system.
We studied a child with a complex skeletal dysplasia who presented with severe scoliosis, thickened calvarium, craniosynostosis, osteosclerosis of the clavicles and spine, and recurring fractures in the lower extremities. We discovered that the disorder was caused by a de novo, neomorphic mutation in SP7, which encodes a transcription factor required for differentiation of osteoblasts. The variant altered the DNA–binding sequence specificity of the transcription factor, causing aberrant expression of osteoblast-related genes, abnormal osteoblast differentiation, and abnormal bone formation.
We also investigated disorders that cause excessive growth. We evaluated a patient with Weaver syndrome, an overgrowth disorder caused by variants in EZH2. EZH2 encodes a histone methyltransferase and thereby acts as an epigenetic writer. We found that the EZH2 variants responsible for Weaver syndrome cause a partial loss of enzymatic function.
We recently studied a child with generalized overgrowth of prenatal onset. Exome sequencing identified a hemizygous frameshift variant in Spindlin 4 (SPIN4, a nuclear protein thought to be involved in histone binding), with X-linked inheritance. Ablation of Spin4 in mice recapitulated the human phenotype with generalized overgrowth, including increased longitudinal bone growth. We found evidence that SPIN4 binds specific histone modifications, promotes canonical WNT signaling, and inhibits cell proliferation in vitro, and that the identified frameshift variant had lost all of these functions. Growth plate analysis revealed increased cell proliferation in the proliferative zone and an increased number of progenitor chondrocytes in the resting zone. We also found evidence of decreased canonical Wnt signaling in growth-plate chondrocytes, providing a potential explanation for the increased number of resting zone chondrocytes. Taken together, our findings provide strong evidence that SPIN4 is an epigenetic reader that negatively regulates mammalian body growth, and that loss of SPIN4 causes an overgrowth syndrome in humans, expanding our knowledge of the epigenetic regulation of human growth.
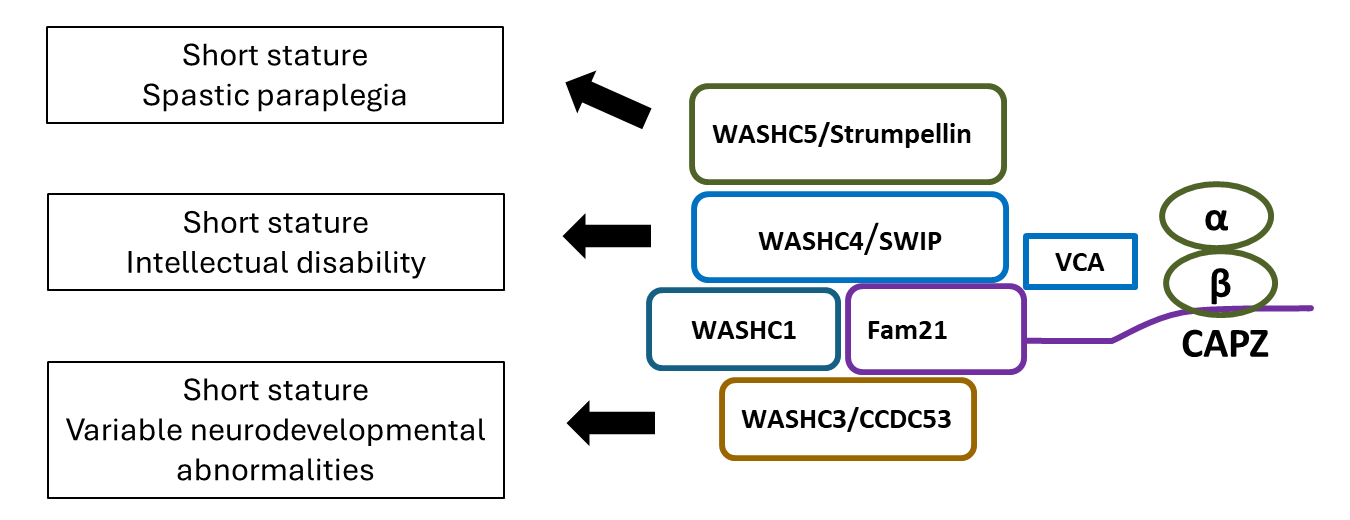
Recently, we studied three families with short stature, neurodevelopmental abnormalities, and distinctive facial appearances. Exome sequencing identified variants in WASHC3, which is involved in endosomal trafficking. We found evidence that the variants affected endosomal trafficking, signal transduction by PTH1R (parathyroid hormone type 1 receptor), and hypertrophic differentiation of growth-plate chondrocytes. In combination with prior studies, our findings indicate that WASHC3 and other members of the WASH complex are required for normal skeletal growth and that, consequently, genetic abnormalities impairing the function of the WASH complex cause short stature as well as distinctive facies and variable neurodevelopmental abnormalities (Figure 2).
Molecular and cellular mechanisms by which specific genes and pathways regulate childhood growth
Our group also studies the fundamental mechanisms governing childhood growth. Much of our work has focused on the growth plate. Growth at the growth plate is controlled by many interacting regulatory systems, involving endocrine, paracrine, extracellular matrix–related, and intracellular pathways. Previously, our group studied growth-plate regulation by FGFs, BMPs, C-type natriuretic peptide, retinoids, WNTs, PTHrP/IHH, IGFs, estrogens, glucocorticoids, and microRNAs. We also found evidence that SOX9, a transcription factor, regulates the trans-differentiation of growth-plate chondrocytes into osteoblasts.
We also investigated the mechanisms that cause bone growth to occur rapidly in early life but then to slow progressively with age and eventually cease. We found evidence that the developmental program responsible for the decline in growth-plate function plays out more slowly in larger bones than in smaller bones and that this differential aging contributes to the disparities in bone length and therefore to establishing normal mammalian skeletal proportions.
New treatment approaches for growth-plate disorders
Recombinant human growth hormone (GH) is commonly used to treat short stature in children. However, GH treatment has limited efficacy, particularly in severe, non-GH–deficient conditions such as chondrodysplasias, and has off-target effects. Systemic insulin-like growth factor-1 (IGF-1) treatment has similar deficiencies. There are many endocrine and paracrine factors that promote chondrogenesis at the growth plate, which could potentially be used to treat these disorders. Targeting these growth factors specifically to the growth plate might augment the therapeutic skeletal effect while diminishing undesirable effects on non-target tissues. To develop growth plate–targeted therapy, we previously used yeast display to identify single-chain human antibody fragments that bind to cartilage with high affinity and specificity. We then created fusion proteins combining these cartilage-targeting antibody fragments with IGF-1, an endocrine/paracrine factor that positively regulates chondrogenesis. Using a GH–deficient mouse model, we found that subcutaneous injections of these fusion proteins increased growth-plate height without increasing proliferation in kidney cortical cells, demonstrating greater on-target efficacy at the growth plate and less off-target effect on the kidney than IGF-1 alone. Our findings provide proof of principle that targeting therapeutics to growth plate cartilage could potentially improve treatment for childhood growth disorders.
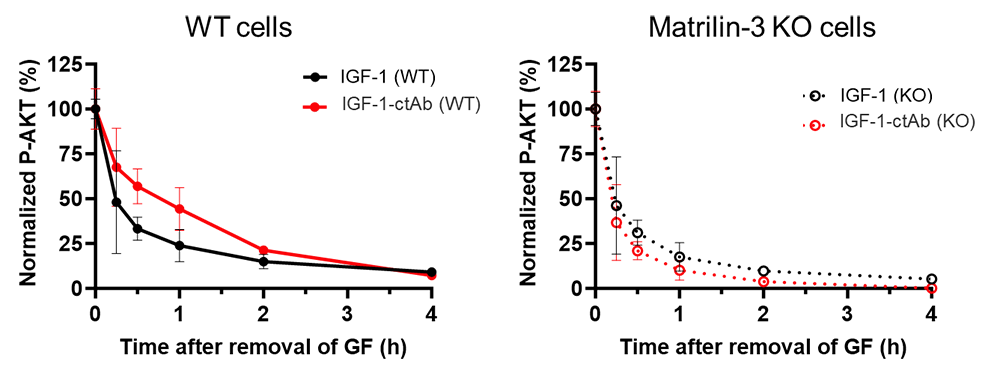
These fusion proteins might be particularly well suited to treat the GH insensitivity (Laron) syndrome. In this disorder, the current treatment with rIGF-1 has incomplete efficacy, substantial adverse effects including hypoglycemia, and requires twice-daily injections. We therefore studied the effects of cartilage-targeted IGF-1 in a mouse model of GH resistance (achieved with pegvisomant). To this end, we created fusion proteins combining cartilage-targeting antibody fragments (ctAb) with IGF-1. We showed that our IGF-1–ctAb fusion proteins had efficacy at the growth plate similar to that seen in the GH–deficient mouse model. A once-daily injection of IGF-1–ctAb showed efficacy at least as great as twice-daily IGF-1 (with twice the daily dose). We also found that subcutaneous IGF-1–ctAb had a lower hypoglycemic effect than IGF-1. In vitro, IGF-1–ctAb caused a prolonged increase in IGF receptor (pAKT) signaling compared with IGF-1, and that this effect was dependent on matrilin-3 (the specific targeted protein) (Figure 3), supporting the hypothesis that matrilin-3 (the specific cartilage matrix protein that is targeted by ctAb) targeting allows IGF-1–ctAb to remain in the proximity of the cell surface for a longer period of time, extending the duration of IGF-1 receptor activation.
Figure 3. IGF-1-ctAb showed prolonged IGF-1 receptor activation in vitro, in the presence of matrilin-3.
Wild-type rat chondrosarcoma (RCS) and matrilin-3 knockout (KO) RCS cells were treated transiently with IGF-1 or IGF-1-ctAb, followed by washout to remove unbound IGF-1 and fusion protein. pAKT was measured in cell lysates at indicated time points.
Figure 3. IGF-1-ctAb showed prolonged IGF-1 receptor activation in vitro, in the presence of matrilin-3.
Wild-type rat chondrosarcoma (RCS) and matrilin-3 knockout (KO) RCS cells were treated transiently with IGF-1 or IGF-1-ctAb, followed by washout to remove unbound IGF-1 and fusion protein. pAKT was measured in cell lysates at indicated time points.
We are also applying this approach to target other chondrogenic endocrine and paracrine factors to the growth plate. We are exploring the utility of this approach both to stimulate growth-plate chondrogenesis non-specifically and also to reverse specific genetic defects in growth plate function by modulating the abnormal molecular pathway responsible for the growth failure.
Additional Funding
- CRADA with Cavalry Biosciences
- NICHD Early Career Award
- NICHD Career Development Award
Publications
- Variants in WASHC3, a component of the WASH complex, cause short stature, variable neurodevelopmental abnormalities, and distinctive facial dysmorphism. Genet Med Open 2024 in press
- Loss of function variant in SPIN4 causes an X-linked overgrowth syndrome. JCI Insight 2023 8:8(9):e167074
- A neomorphic variant in the transcription factor SP7 alters sequence specificity and causes a high-turnover bone disorder. Nat Commun 2022 13:700
- Epigenetic causes of overgrowth syndromes. J Clin Endocrinol Metab 2024 109:312-332
- Identification of novel genes including NAV2 associated with isolated tall stature. Front Endocrinol (Lausanne) 2023 14:1258313
Collaborators
- Lijin Dong, PhD, Genetic Engineering Core, NEI, NIH, Bethesda, MD
- Gabriele Häusler, MD, Medical University of Vienna, Austria
- Youn Hee Jee, MD, Children’s National Medical Center, Washington DC
- Ola Nilsson, MD, PhD, Karolinska Institute, Stockholm, Sweden
- Gudrun Rappold, PhD, University of Heidelberg, Heidelberg, Germany
Contact
For more information, email jeffrey.baron@nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/baron.