Quantitative Imaging and Tissue Sciences
- Peter J. Basser,
PhD, Head, Section on Quantitative Imaging and Tissue Sciences - Ferenc Horkay, PhD, Staff Scientist
- Julian Rey, PhD, Postdoctoral Research Associate Training (PRAT) Fellow
- Nathan Williamson, PhD, Postdoctoral Intramural Research Training Award Fellow
- Teddy Cai, BS, Postdoctoral Intramural Research Training Award Fellow
- Ella Wilczynski, PhD, Postdoctoral Visiting Fellow
- Hiya Sawhney, BS, Postbaccalaureate Intramural Research Training Associate; Special Volunteer
- Alexandru V. Avram, PhD, Collaborating Contract Scientist
- Rea Ravin, PhD, Collaborating Contract Scientist
- Kadharbatcha Saleem, PhD, Collaborating Contract Scientist
- Mihilka Gangolli, PhD, Collaborating Scientist funded via the Henry Jackson Foundation and the Military Traumatic Brain Injury Initiative
- Michal Komlosh, PhD, Collaborating Scientist funded via the Henry Jackson Foundation and the Military Traumatic Brain Injury Initiative
- Magdoom Kulam, PhD, Collaborating Scientist funded via the Henry Jackson Foundation and the Military Traumatic Brain Injury Initiative
- R. D. Fields, PhD, Special Volunteer
- Iren Horkayne-Szakaly, MD, Special Volunteer
- Shinjini Kundu, MD, PhD, Special Volunteer
- Steven Suomi, PhD, Special Volunteer
- Velencia Witherspoon, PhD, Special Volunteer

In our tissue-sciences research, we are interested in how tissue microstructure, hierarchical organization, composition, and material and transport properties all work together to affect biological function or dysfunction. We investigate biological and physical model systems at various length and time scales, performing biophysical measurements and developing novel physical/mathematical models (including molecular dynamics [MD] and continuum models) to explain their functional properties and behavior. We use water to probe tissue structure and function from lengths of nanometers to centimeters and from times of microseconds to lifetimes. Our armamentarium includes small-angle neutron scattering (SANS), small-angle X-ray scattering (SAXS), static light scattering (SLS), dynamic light scattering (DLS), atomic force microscopy (AFM), osmometry, and multi-dimensional nuclear magnetic resonance (NMR) methods. We develop research tools that can be translated from bench-based quantitative methodologies to bedside biomarkers to aid in diagnosis, prognosis assessment, and therapy.
Our work is directed toward making critical ‘invisible’ biological structures and processes ‘visible.’ Our quantitative imaging group uses knowledge of physics, engineering, applied mathematics, imaging, and computer sciences, as well as key insights gleaned from our tissue-sciences research, to discover, vet, and develop novel quantitative imaging biomarkers that can detect changes in tissue composition, microstructure, and/or microdynamics with high sensitivity and specificity so as to assess normal and abnormal developmental trajectories, diagnose childhood diseases and disorders, and characterize degeneration and trauma, such as mild traumatic brain injury (mTBI). MRI is our imaging modality of choice because it is non-invasive, non-ionizing, usually requires no exogenous contrast agents or dyes, and is generally deemed safe and effective for use with mothers, their fetuses, and children in both clinical and research settings. Critical to this enterprise is our ability to follow water as it migrates through complex media as a probe of its microstructure, and to assess water's interactions with biomolecules to identify distinct water compartments in tissues.
Virtual in vivo MRI histology
Our most mature in vivo MRI technology is Diffusion Tensor MRI (DTI), by which we measure and map D, a diffusion tensor of water, throughout an imaging volume, often the entire human brain. In neuroradiology, the mean apparent diffusion constant (mADC) is the most widely used diffusion imaging parameter to identify ischemic areas in the brain during acute stroke, to diagnose cancer, and to follow the patient's response to cancer therapy. The mADC is becoming increasingly used to diagnose cancers, such as multiple myeloma, and assess disease progression and response to therapy. Measures of diffusion anisotropy we first proposed (e.g., the fractional anisotropy or FA) are widely used to follow changes in normally and abnormally developing white matter and in many other clinical and neuroscience applications, such as brain white-matter visualization. Our group also pioneered the use of fiber direction–encoded color (DEC) maps to display the orientation of white matter pathways in the brain. To assess anatomical connectivity among various cortical and deep-brain gray-matter areas, we also developed DTI ‘Streamline’ Tractography, which is used by neuroscientists to track white-matter pathways to help establish ‘anatomical connectivity,' to help neurosurgeons plan brain surgeries, and radiation oncologists to plan radiation dosing, so as to spare ‘eloquent’ areas of the brain. Advances in medical imaging from our lab also helped inspire several large federally funded research initiatives, including the NIH Human Connectome Project (HCP) and, more recently, the NIH Brain Initiative.
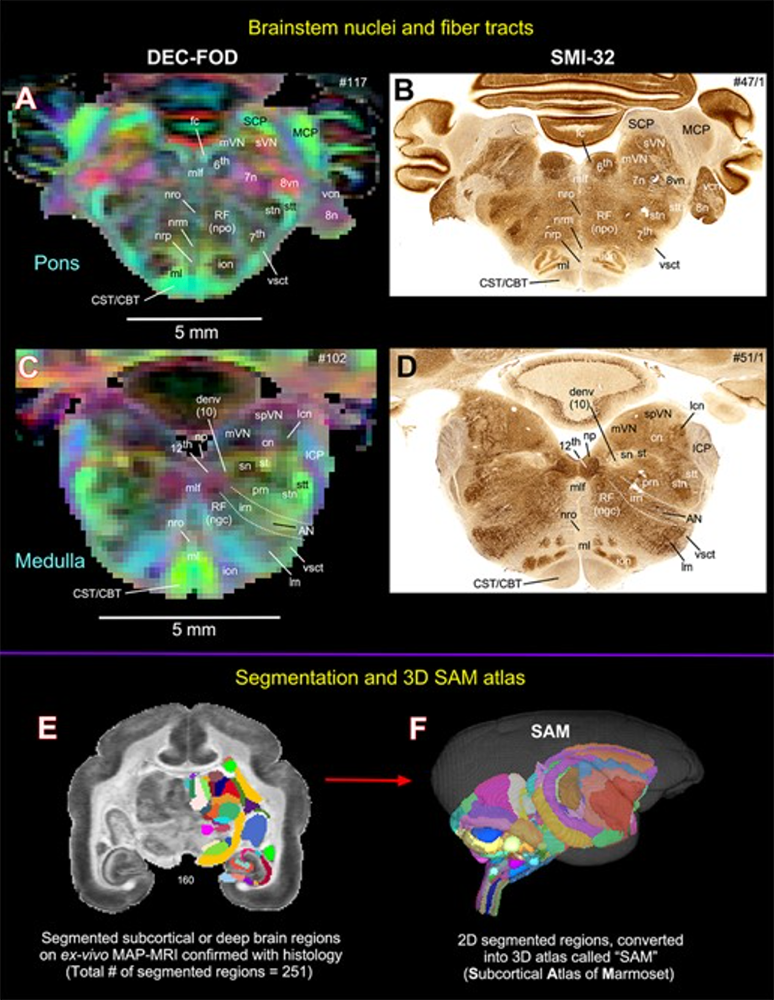
Figure 1. Atlas of marmoset brainstem obtained via diffusion MRI and histology
Mean Apparent Propagator (MAP) MRI data obtained in marmoset brainstem show remarkable agreement with histologically stained sections of the same specimen. Diffusion encoded color (DEC)–fiber orientation distribution (FOD) images show both the local axonal orientation and help delineate and distinguish nearby structures. A and C contain MAP-MRI–derived DEC-FOD images that show agreement with corresponding regions identified in B and D, obtained using histological staining techniques. E: coronal image of segmented deep brain regions; F: sagittal image of the segmented subcortical atlas of the Marmoset (SAM).
Figure 1. Atlas of marmoset brainstem obtained via diffusion MRI and histology
Mean Apparent Propagator (MAP) MRI data obtained in marmoset brainstem show remarkable agreement with histologically stained sections of the same specimen. Diffusion encoded color (DEC)–fiber orientation distribution (FOD) images show both the local axonal orientation and help delineate and distinguish nearby structures. A and C contain MAP-MRI–derived DEC-FOD images that show agreement with corresponding regions identified in B and D, obtained using histological staining techniques. E: coronal image of segmented deep brain regions; F: sagittal image of the segmented subcortical atlas of the Marmoset (SAM).
More recently, we invented and developed a family of advanced in vivo diffusion MRI methods to measure fine-scale microstructural features of axons and other central nervous system (CNS) components (also known as ‘microstructure imaging’), which otherwise could only be assessed using ex vivo histological or pathological methods. These features are generally orders of magnitude smaller than the imaging voxel, but can still be fleshed out using the migration of water molecules to “palpate” them. For example, we have been developing efficient means for performing ‘k- and q-space MRI’ in the living human brain, such as ‘Mean Apparent Propagator’ (MAP) MRI, an approach that can detect subtle microstructural and architectural features in both gray and white matter at the micron scale. MAP MRI also subsumes DTI, as well as providing new in vivo quantitative imaging biomarkers to measure and map. We also developed CHARMED MRI, which measures the average axon diameter (AAD), and AxCaliber MRI, which measures the axon-diameter distribution (ADD) along white-matter pathways. The ADD is functionally important, given that axon diameter is a critical determinant of action potential conduction velocity and therefore the rate at which information can be transmitted along axon bundles throughout the brain. The ADD also helps to determine the latencies or time delays of neural impulses between and among different connected brain areas. This led us to propose a novel MRI–based method to measure the ‘latency connectome,’ including a latency matrix that reports conduction times between different brain areas. We also derived a statistical distribution to explain the form of the observed ADDs in different fascicles, suggesting that they represent a trade-off between maximizing information flow and minimizing metabolic demands. We developed novel multiple pulsed-field gradient (mPFG) methods and demonstrated their feasibility in vivo on conventional clinical MRI scanners as a further means to extract quantitative features in the CNS, such as the AAD and other features of cell-size distribution and cell shape.
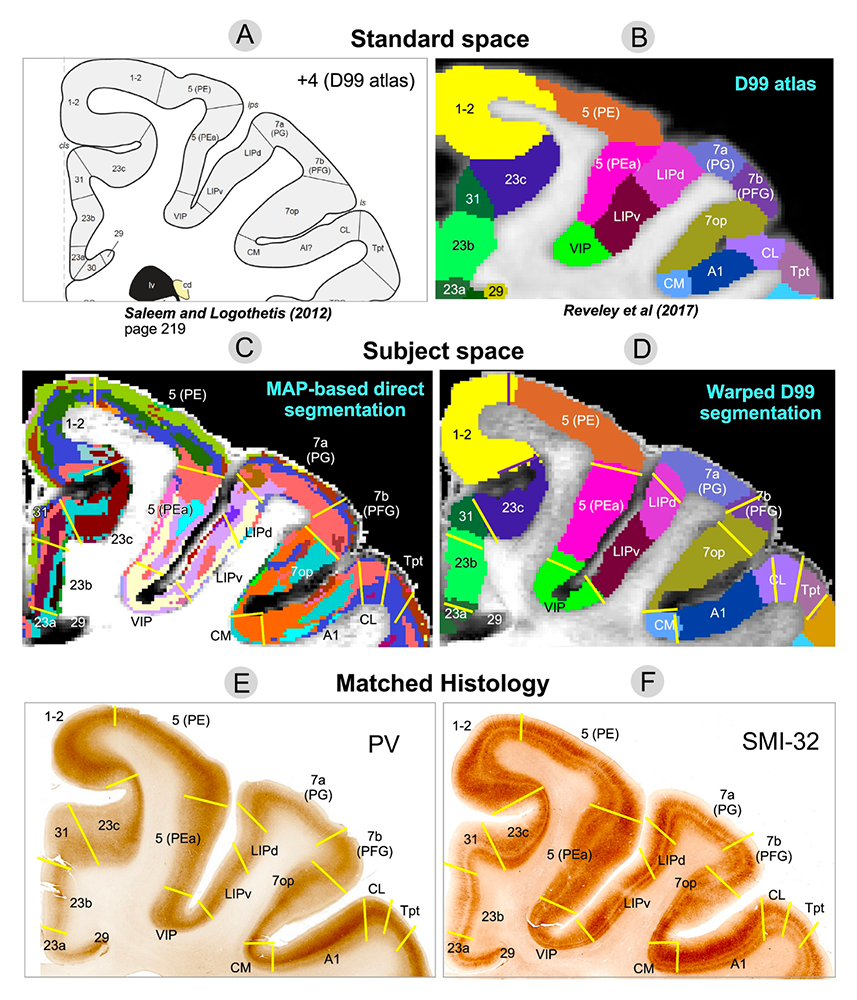
Figure 2. Toward in vivo cortical parcellation in the human brain using advance diffusion MRI data
Mean Apparent Propagator (MAP) MRI helps delineate both cortical areas and layers in fixed brains with the same reliability and quality as using histological staining methods, which require laborious tissue processing steps following tissue removal.
Figure 2. Toward in vivo cortical parcellation in the human brain using advance diffusion MRI data
Mean Apparent Propagator (MAP) MRI helps delineate both cortical areas and layers in fixed brains with the same reliability and quality as using histological staining methods, which require laborious tissue processing steps following tissue removal.
Although gray matter appears featureless using DTI, its microstructure and architecture are rich and varied throughout the brain, both along the brain's cortical surface and within the brain's deep gray-matter regions. To target this tissue, we have been developing several non-invasive, in vivo methods to measure features of cortical gray-matter microstructure and architectural organization that are plainly visible in electron micrographs (EM) but currently invisible in conventional MRI. One example is diffusion tensor distribution (DTD) MRI, in which we use a novel normal tensor-variate distribution to characterize heterogeneity within complex tissue voxels. One of our long-term goals is to segment the cerebral cortex in vivo into its approximately 500 distinct cyto-architectonic areas using non-invasive imaging methods by developing advanced MRI sequences and analysis pipelines to assess variability in tissue properties in the cortex, such as CORTEC, and others that probe correlations among relaxivities and diffusivities of different water pools. In the latter case, we use multi-dimensional MRI relaxometry and diffusometry to study water mobility and diffusion simultaneously in gray and white matter. We continue to work to translate these and other methods to the clinic to help identify changes in normal and abnormal development, as well as in inflammation and trauma by developing radiological-pathological correlations between MR and neuropathological images of TBI tissue specimens as a way to identify potential quantitative imaging biomarkers of injury or inflammation that have the potential to detect TBI in vivo. We are in the process of migrating many of these methods both to pre-clinical and clinical applications under the auspices of the Military Traumatic Brain Injury Initiative (MTBI2) through a collaboration among the Uniformed Services University of Health Sciences (USUHS), the Henry Jackson Foundation (HJF), NICHD, and NIA.
Teddy Cai has recently developed a means to measure the powerful new quantitative imaging biomarker, the velocity autocorrelation function (VACF) via MRI, to probe water migration behavior in tissues and cells. Kadharbatcha Saleem and Alexandru Avram have published an atlas of the marmoset monkey brain based on high-resolution MAP MRI and histology, advancing our goal of performing virtual in vivo whole-brain histology.
Quantitative MRI biomarker research and development
Clinical MRI still lacks the quantitative character of computer tomography (CT) imaging, revealing only either gross morphological features or focal abnormalities, but has benefited greatly from the introduction of ‘quantitative’ clinical MRI techniques, with which one measures and maps meaningful intrinsic physical quantities or chemical variables that possess physical units and can be compared among different tissue regions. Quantitative MRI methods such as DTI also increase sensitivity, providing a basis for monitoring subtle changes that occur, e.g., during the progression or remission of disease. Quantitative MRI methods should continue to advance ‘precision imaging’ studies, in which MRI phenotypic and genotypic data can be meaningfully incorporated and used for improved diagnosis and prognosis assessments, such as in the NIH ENIGMA (Enhanced NeuroImaging Genetics through MetaAnalysis) project.
We have developed numerical and statistical methods, including algorithms that generate a continuous, smooth approximation to the discrete, noisy, measured DTI field data, so as to reduce noise, and which allowed us to implement ‘Streamline’ Tractography. We proposed a novel Gaussian distribution for the tensor-valued random variables that we use to design optimal DTI experiments and interpret their results. We and others recently repurposed this framework to characterize heterogeneity of diffusion properties within individuals voxels. In tandem, we developed non-parametric empirical (e.g., Bootstrap) methods to determine the statistical distribution of DTI–derived quantities in order to study, e.g., the variability and reliability of computed white-matter trajectories, enabling us to assess the statistical significance of findings in a wide range of biological and clinical applications that had previously been tested using ad hoc statistical methods. We are also developing novel methods to register different brain volumes and to generate group-averaged DTI data or atlases from various subject populations, based on the Kullback-Leibler divergence and other distance metrics, like the “earthmover's distance.”
Previously, we carried out clinical studies that utilize novel quantitative MRI acquisition and analysis methods and whose aim is to improve accuracy and reproducibility of diagnosis and to detect and follow normal and abnormal development children and adolescents. One early example is the NIH Study of Normal Brain Development, jointly sponsored by the NICHD, NIMH, NINDS, and NIDA, which was initiated in 1998. The Brain Development Cooperative Group (archived June 24, 2024) is still publishing, primarily by mining the MRI data, many of which our lab processed and contributed, serving as the study's DTI Data-Processing Center (DPC). The processed DTI data were uploaded to the National Database for Autism Research (NDAR). Our former Staff Scientist Carlo Pierpaoli, who spearheaded this work, continues to support, update, and disseminate the processing and analysis software called “TORTOISE,” which grew out of our efforts and can be downloaded from http://www.tortoisedti.org. We continue to improve our ability to follow normal trajectories in pediatric brain development through an active collaboration between the Gates Foundation (GF) and NICHD. Our role in this UNITY project is to develop new MRI pulse sequences and analysis pipelines to probe the state of different water populations in tissue, which has the potential to follow normal and abnormal pediatric brain developmental trajectories.
Traumatic Brain Injury (TBI) represents a significant public health challenge for our pediatric population, but also for those serving in the military. Our TBI research, particularly in detecting mild TBI (mTBI), has continued to expand through partnerships with various Department of Defense (DoD) entities and the GE-NFL-mTBI MRI project. Diffusion MRI (dMRI) provides essential information to aid in the assessment of TBI, but conventional dMRI methods have lacked sufficient specificity. To improve the accuracy and reproducibility of dMRI we developed a data-processing pipeline, and, in collaboration with the DoD Military Traumatic Brain Injury Initiative (MTBI 2), we helped incorporate MAP-MRI into our NICHD TORTOISE pipeline to detect sequelae of mTBI. We have also been employing multi-dimensional MRI relaxometry-diffusometry methods to study the etiology of various types of TBI, in collaboration with the USUHS Neuropathology Research Division and under the auspices of MTBI 2, and to improve the correlation and integration of neuropathology and neuro-radiological imaging data. We also partnered with MTBI 2 to study ways to measure very slow flows that occur during glymphatic transport, a mechanism the brain uses to wash away harmful macromolecules. With our partners at the University of Arizona, this pre-clinical research is providing experimental data to enable us to migrate these imaging approaches to the clinic.
Figure 4. Potential MRI means to detect mild traumatic brain injury (mTBI)
In a CRADA with General Electric we are able to analyze a tranche of MRI data they collected under the auspices of the GE-NFL-mTBI-MRI study. The experimental design admits several different post-processing analysis methods. Initially we tried several, including our own diffusion tensor MRI (DTI), but none showed a “smoking gun,” i.e., brain regions in which radiological analysis pointed to a possible injury site. We then measured and mapped quantitative imaging biomarkers obtained from MAP-MRI, with promising findings in some interfacial areas, regions between different tissue types in the brain, where one might expect damage to occur owing to differences in mechanical impedance across these boundaries. Hyperintensities were found in several such areas using MAP-MRI–derived imaging biomarkers. We plan to use our image processing pipeline on a larger data set acquired under the auspices of the GE-NFL-mTBI-MRI study to see whether we can definitively identify mTBI quantitative imaging biomarkers.
Figure 4. Potential MRI means to detect mild traumatic brain injury (mTBI)
In a CRADA with General Electric we are able to analyze a tranche of MRI data they collected under the auspices of the GE-NFL-mTBI-MRI study. The experimental design admits several different post-processing analysis methods. Initially we tried several, including our own diffusion tensor MRI (DTI), but none showed a “smoking gun,” i.e., brain regions in which radiological analysis pointed to a possible injury site. We then measured and mapped quantitative imaging biomarkers obtained from MAP-MRI, with promising findings in some interfacial areas, regions between different tissue types in the brain, where one might expect damage to occur owing to differences in mechanical impedance across these boundaries. Hyperintensities were found in several such areas using MAP-MRI–derived imaging biomarkers. We plan to use our image processing pipeline on a larger data set acquired under the auspices of the GE-NFL-mTBI-MRI study to see whether we can definitively identify mTBI quantitative imaging biomarkers.
In a long-term collaboration with Sara Inati, who studies focal epilepsy, a devastating disorder that is difficult to detect using conventional neuroradiological methods, we are developing and testing various new MRI–based methods that we believe may reveal pathological changes in brain microstructure and architectural organization in this disorder, for example in cortical dysplasia, to improve localization and assessment of cortical lesions. We are currently testing the utility of DTD MRI approach on Sara Inati's patient cohort.
Other quantitative biomarkers we are developing entail characterizing different material or mechanical properties of brain tissue in vivo. Magdoom Kulam developed a novel MRI pipeline, magnetic resonance elastography (MRE), to measure the distribution of tissue stiffness throughout the brain without the use of a mechanical actuator, a method that was tested before tissue and clinical tests were performed. Recently, this approach was tested in vivo in normal human subjects.
Biopolymer physics: water-ion-biopolymer interactions
Despite their crucial role in biological processes, little is still known about the physics of water-ion-biopolymer interactions in the physiological ionic-strength range. To determine the effect of ions on the structure and dynamics of key biopolymers, we combined macroscopic techniques (osmotic swelling-pressure and mechanical measurements) with high-resolution scattering methods (e.g., SANS and SAXS, SLS and DLS). Macroscopic swelling-pressure measurements provide information about the overall thermodynamic response of the system, while SANS, SAXS, SLS and DLS probe biopolymers at molecular and atomic through supramolecular length scales to quantify the effect of changes in the environment (e.g., ion concentration, ion valance, pH, temperature) on the structure and interactions among biopolymers, water, and ions.
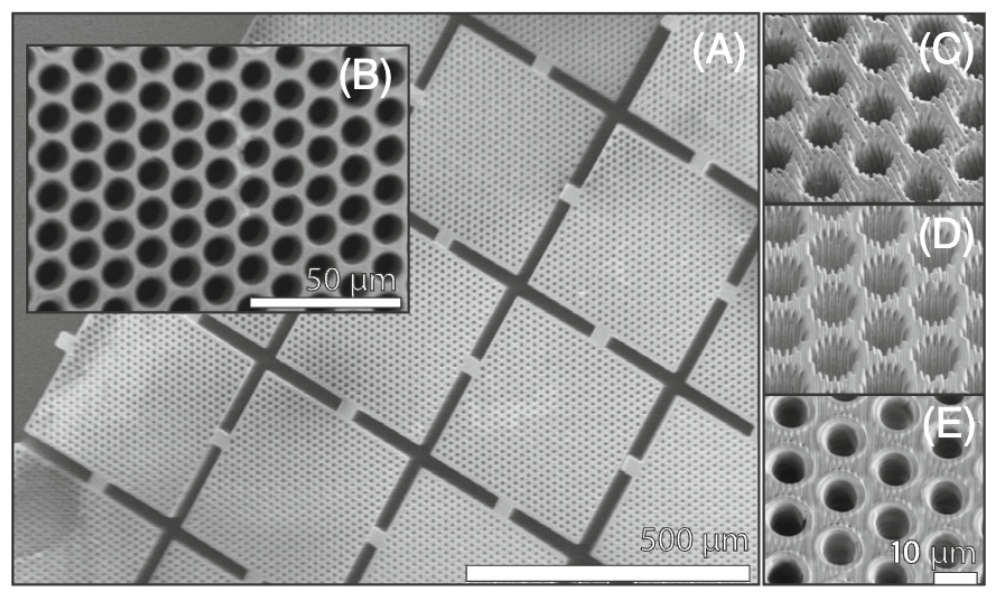
These basic studies on biomimetic tissue analogs have led to the development of novel MRI phantom designs to support our quantitative imaging program, including diffusion MRI phantoms, which we use to calibrate scanners to assure the quality and fidelity of imaging data in single-subject, longitudinal, and multi-site studies. For instance, our U.S. Patent for a ‘Phantom for diffusion MRI imaging’ is now incorporated in CaliberMRI phantoms, enabling quantitative diffusion MRI studies at many clinical sites worldwide. Our colleagues at NIST Boulder have incorporated our polyvinylpyrrolidone (PVP) polymer into their own diffusion MRI NIST “standard”. We used various glass microcapillary array (GCA) geometries to mimic features of axons in white-matter pathways in order to interrogate our AxCaliber nd dPFG MRI models of water diffusion in such pathways. Valencia Witherspoon developed a variety of NMR and MRI phantoms, such as a 3-D printed polymeric phantom, which possess various features of cell or tissue systems, as well as multi-component polymer gel systems for use as MRI phantoms exhibiting water exchange to calibrate our novel Diffusion Exchange Spectroscopy (DEXSY) MRI acquisitions.
Measuring and mapping functional properties of extracellular matrix
We study interactions among the main extracellular matrix (ECM) components, initially using cartilage as a model system because it is aneural, avascular, and nearly acellular. In cartilage ECM, collagen (type II) is organized into fiber bundles that form a network that entraps the major proteoglycan (PG), the bottlebrush-shaped aggrecan molecule. The biomechanical behavior of cartilage and other ECMs reflects their molecular composition and microstructure, which change during development, disease, degeneration, and aging. To determine tissue structure/function relationships, we measured physical/chemical properties of ECM tissues and tissue analogs at different length and time scales, using a variety of complementary static and dynamic experimental techniques, e.g., osmometry, SANS, SAXS, neutron spin-echo (NSE), SLS, DLS, and AFM. Understanding the physical and chemical mechanisms affecting cartilage swelling (hydration) is essential to predicting its load-bearing and lubricating ability, which are mainly governed by osmotic and electrostatic forces. To quantify the effect of hydration on cartilage properties, we previously developed a novel tissue micro-osmometer to measure the effect of equilibrium water activity (vapor pressure) in small tissue samples (less than 1 µg). We also make osmotic-pressure measurements to determine how the individual components of cartilage ECM (e.g., aggrecan and collagen) contribute to the total load-bearing capacity of the tissue. We also demonstrated that aggrecan-hyaluronic aggregates self-assemble into microgel particles, contributing to improved dimensional stability and lubricating ability of the tissue. We found that aggrecan is highly insensitive to changes in the ionic environment, particularly to divalent cations such as calcium, which is critical for maintaining the tissue's mechanical integrity and allowing aggrecan to serve as a calcium-ion reservoir in cartilage and bone.
More recently, to model cartilage ECM, Ferenc Horkay's group invented and developed a new biomimetic composite material consisting of polyacrylic acid (PAA) µgel particles dispersed and embedded within a polyvinyl alcohol (PVA) fibrous gel network. PAA mimics cartilage's proteoglycan phase (i.e., hyaluronic acid-aggrecan complexes), while PVA mimics the fibrous collagen network phase entrapping them. The PVA/PAA biomimetic composite model system reproduces both the shape of the cartilage-swelling pressure curves and the stiffness values reported for healthy and osteoarthritic human cartilage specimens. Studies on these model composite hydrogels yield invaluable insights into how macromolecular factors (matrix stiffness, swelling pressure, fixed-charge density, etc.) can affect the tissue's macroscopic mechanical/swelling properties, and ultimately its load-bearing and lubricating abilities, and their loss in various diseases and disorders, including osteoarthritis.
We are now attempting to develop and design novel non-invasive MRI methods, with the aim of inferring parameters characterizing ECM composition, patency, and functional properties in vivo. Our goal is to use MRI for early diagnosis of diseases of cartilage and other tissue and organs to follow normal and abnormal ECM development, which entails making components of ECM (e.g., collagen and PGs) that are ‘invisible’ to MR ‘visible’ so as to predict the functional properties of the composite tissue, such as its load-bearing ability. An obstacle is that protons bound to immobile species (e.g., collagen) are largely invisible with conventional MRI methods. However, magnetization exchange (MEX) MRI (as well as other related methods) now make it possible to detect the bound protons indirectly by transferring their magnetization to the abundant free water protons surrounding them, which enables us to quantitate collagen content in tissue. In previous pilot studies with Uzi Eliav (deceased) and Ed Mertz, we applied the new MEX MRI method to determine the concentration and distribution of the main macromolecular constituents in bovine femoral-head cartilage samples. The results were qualitatively consistent with those obtained by histological techniques, such as high-definition infrared (HDIRI) spectroscopic imaging. Our approach has the potential to map tissue structure and functional properties in vivo and non-invasively. More recently, we developed molecular dynamics (MD)–based models of cartilage and cartilage ECM analogs in order to interpret our experimental findings in terms of molecular interactions and processes. Currently, we are using Finite Element Models (FEM) to describe the behavior of these novel composite materials to explain and predict their properties.
We have also been employing several novel MR methodologies to characterize ECM properties using our one-sided NMR systems to study water relaxation, diffusion, and exchange processes in various tissue specimen. Most recently, Velencia Witherspoon used these approaches to study the organization and structure of fascia. Moreover, we are adapting these methods to study the composition and organization of brain ECM, which is a complex medium about which surprisingly little is known. There is even disagreement about the composition and molecular architecture of brain ECM. We hope to make this invisible component visible using various novel MRI methods under development.
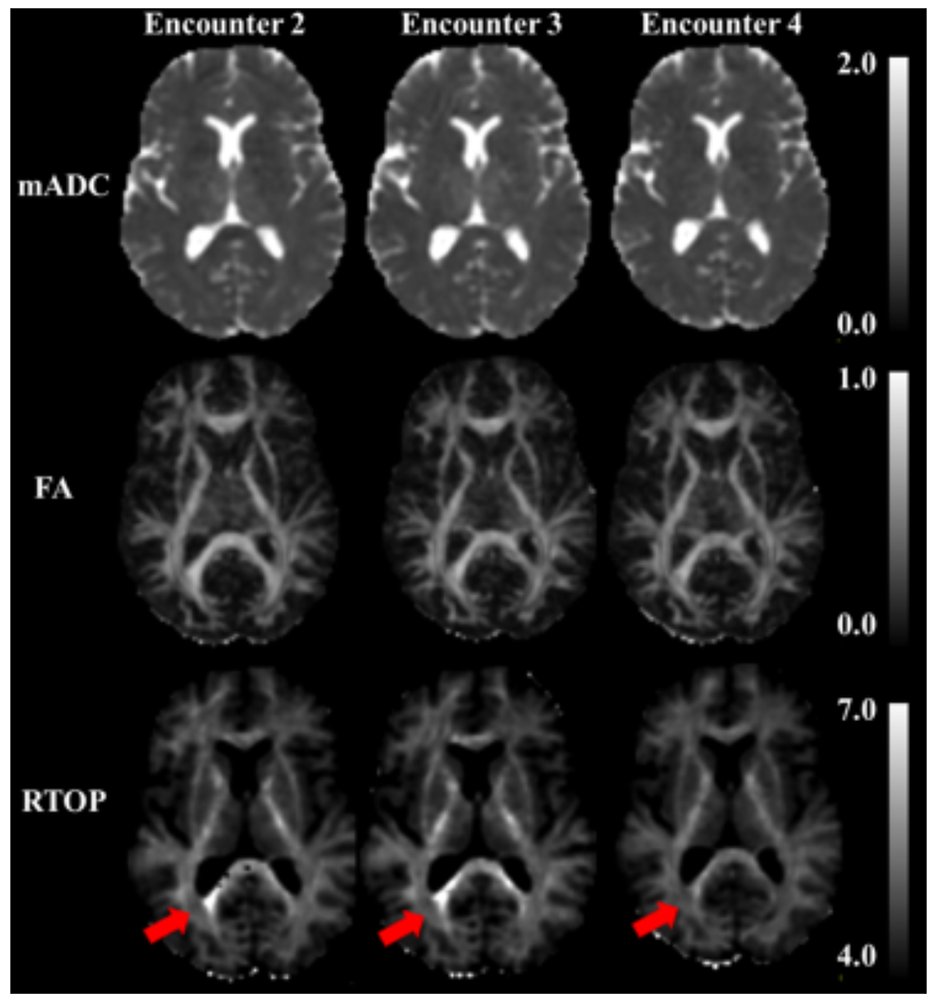
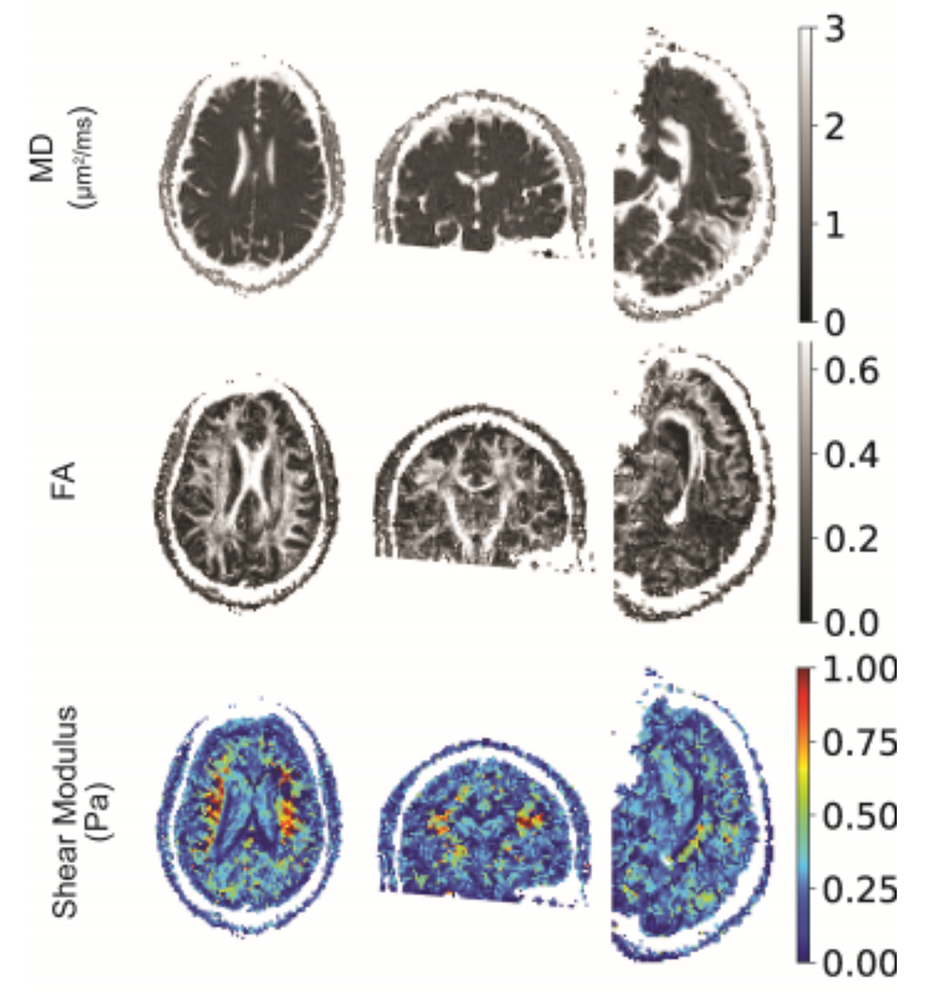
Figure 5. In vivo tamperless magnetic resonance elastography (MRE) in the human brain
The stiffness of brain tissue (parenchyma) allows it to absorb mild shocks and vibrations, but when the brain is injured, changes in its material properties can occur. We developed a novel method to measure and map material parameters describing the brain's mechanical properties non-invasively via MRI, as a means to monitor “normal” tissue properties and possibly as a diagnostic to monitor tissue abnormalities. Current methods to perform magnetic resonance elastography (MRE) entail using a tamper or actuator to excite mechanical waves in tissue, but this is not indicated in individuals with a traumatic brain injury or with pediatric or obstetric patients. The top row shows the mean apparent diffusion coefficient (mADC) in a brain slice. The middle row shows the fractional anisotropy (FA), a widely used diffusion tensor MRI parameter that helps identify white-matter pathways in the brain, and below these, is a material stiffness map. Although somewhat noisy, the map distinguishes between between gray and white matter, in which the stiffness is greater. We expect improvements in image quality and accuracy as we continue to develop this method.
Figure 5. In vivo tamperless magnetic resonance elastography (MRE) in the human brain
The stiffness of brain tissue (parenchyma) allows it to absorb mild shocks and vibrations, but when the brain is injured, changes in its material properties can occur. We developed a novel method to measure and map material parameters describing the brain's mechanical properties non-invasively via MRI, as a means to monitor “normal” tissue properties and possibly as a diagnostic to monitor tissue abnormalities. Current methods to perform magnetic resonance elastography (MRE) entail using a tamper or actuator to excite mechanical waves in tissue, but this is not indicated in individuals with a traumatic brain injury or with pediatric or obstetric patients. The top row shows the mean apparent diffusion coefficient (mADC) in a brain slice. The middle row shows the fractional anisotropy (FA), a widely used diffusion tensor MRI parameter that helps identify white-matter pathways in the brain, and below these, is a material stiffness map. Although somewhat noisy, the map distinguishes between between gray and white matter, in which the stiffness is greater. We expect improvements in image quality and accuracy as we continue to develop this method.
Patents
- Peter Basser, Kulam Najmudeen, Magdoom Mohamed. Tamperless Tensor Elastography Imaging. (PDF 1 MB) PCT/US2022/030846
- Ferenc Horkay, Peter Joel Basser. Composite gels and methods of use thereof. US Patent #12,083,246.
- Peter J. Basser, Alexandru V. Avram. Isotropic generalized diffusion tensor MRI. US Patent 11835611, filed April 6, 2018, and issued December 5, 2023.
- Dan H. Benjamini, Peter J. Basser, D. Iacono. Multidimensional mri signature for specific detection of traumatic brain injury in vivo. US Patent App. 18/019,725
- Peter J. Basser, Dan H Benjamini. Multi-dimensional spectroscopic NMR and MRI using marginal distributions. US Patent 11,846,690
- Magdoom Mohamed Kulam Najmudeen, Peter J. Basser, Michal E. Komlosh. Time efficient multi-pulsed field gradient (mpfg) mri without concomitant gradient field artifacts. Application #20230266418.
Additional Funding
- “Connectome 2.0: Developing the next generation human MRI scanner for bridging studies of the micro-, meso- and macro-connectome.” NIH BRAIN Initiative-funded 1U01EB026996-01
- “Advanced Neuroimaging Research and Development Core” MTBI2/USUHS/HJF/DoD
Publications
- Direct segmentation of cortical cytoarchitectonic domains using ultra-high-resolution whole-brain diffusion MRI. bioRxiv 2024 preprint
- Novel pore size-controlled, susceptibility matched, 3D-printed MRI phantoms. Magn Reson Med 2024 1–12
- “Tumor Treating Fields” delivered via electromagnetic induction have varied effects across glioma cell lines and electric field amplitudes. Am J Cancer Res 2024 14:562–584
- The Subcortical Atlas of the Marmoset (“SAM”) monkey based on high-resolution MRI and histology. Cerebr Cortex 2024 34:bhae120
- The Diffusion Exchange Ratio (DEXR): A minimal sampling of diffusion exchange spectroscopy to probe exchange, restriction, and time-dependence. J Magn Reson 2024 366:107745
- Cartilage extracellular matrix polymers: hierarchical structure, osmotic properties, and function. Soft Matter 2024 20:6033–6043
Collaborators
- Dan Benjamini, PhD, Unit on Multiscale Imaging and Integrative Biophysics, NIA, Bethesda, MD
- Jack Douglas, PhD, Materials Science and Engineering Division, NIST, Gaithersburg, MD
- David Feinberg, PhD, University of California, Berkeley, CA
- Dario Gasbarra, PhD, University of Helsinki, Helsinki, Finland
- Silvina Horowitz, PhD, Mobile Clinical Research Unit, NINDS, Bethesda, MD
- Mark R. Gilbert, MD, Neuro-Oncology Branch, Center for Cancer Research, NCI, Bethesda, MD
- Susie Huang, MD, PhD, Martinos Center, Harvard Medical School, Boston, MA
- Beth Hutchinson, PhD, University of Arizona, Tucson, AZ
- Sara Inati, MD, Electroencephalography (EEG) Section, NINDS, Bethesda, MD
- Edward Mertz, PhD, Section on Physical Biochemistry, NICHD, Bethesda, MD
- Nicole Y. Morgan, PhD, Trans-NIH Shared Resource on Biomedical Engineering and Physical Science (BEPS), NIBIB, Bethesda, MD
- Michael O'Donovan, MD, PhD, Developmental Neurobiology Section, NINDS, Bethesda, MD
- Evren Özarslan, PhD, Linköping University, Linköping, Sweden
- Sinisa Pajevic, PhD, Section on Critical Brain Dynamics, NIMH, Bethesda, MD
- Daniel Perl, MD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Carlo Pierpaoli, MD, PhD, Laboratory on Quantitative Medical Imaging, NIBIB, Bethesda, MD
- Dietmar Plenz, PhD, Section on Critical Brain Dynamics, NIMH, Bethesda, MD
- Randall Pursley, MS, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Joelle Sarlls, PhD, In Vivo NMR Center, NINDS, Bethesda, MD
- Brain Development Cooperative Group, Various
Contact
For more information, email basserp@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/basser.