Biophysics of Large Membrane Channels
- Sergey M. Bezrukov,
PhD, DSci, Head, Section on Molecular Transport - Tatiana K. Rostovtseva, PhD, Associate Scientist
- Megha Rajendran, PhD, Visiting Fellow
- Wendy Fitzgerald, BS, Biologist
- Antara Syam, BS, Graduate Partnerships Program Predoctoral Fellow
- William Augustus Milhizer, BS, Postbaccalaureate Fellow
- Mya Wolfe, BA, Postbaccalaureate Fellow
- Alexander M. Berezhkovskii, PhD, Contractor

We study the functional properties of mitochondrial and bacterial membrane proteins that form “large” beta-barrel channels. Such channels are the gateways of metabolite exchange as well as the components of many toxins. Importantly, they are implicated in the pathophysiology of most common diseases and recognized as promising pharmacological targets. For example, the voltage-dependent anion channel (VDAC) from the outer membrane of mitochondria was shown to be involved in cancer, stroke, heart attack, Parkinson's and Alzheimer's neurodegenerative disorders, and aging. Our motivation is that learning the molecular mechanisms of channel operation is vital for developing new approaches to treat various diseases in which regulation of transport through ion channels plays a key role. The benchmark approach of our lab is channel reconstitution into planar lipid membranes, a technique that we complement with the physical theory of transport and different biophysical and cell biology methods. Currently, we mainly focus on VDAC, but other beta-barrel channels, such as MspA (major outer-membrane porin from Mycobacterium smegmatis), alpha-Hemolysin (toxin from Staphylococcus aureus), OmpF (general bacterial porin from Escherichia coli), LamB (sugar-specific bacterial porin from Escherichia coli), OprF (porin from Pseudomonas aeruginosa), translocation pores of B. anthracis (PA63), C. botulinum (C2IIa), and C. perfringens (Ib) binary toxins, are also within the scope of our interest. Though these channels differ by their primary structure and are responsible for drastically different biological functions, they have similar beta-barrel motifs and biophysical properties, so their comparative analysis provides an in-depth understanding of the underlying transport mechanisms.
To investigate channels under precisely controlled conditions and, if necessary, at the single-molecule level, we first isolate the corresponding channel-forming proteins from their host organisms (or, alternatively, refold recombinant proteins from inclusion bodies), purify, and then insert them into planar lipid membranes for electrophysiological examination. Our main goal is to elucidate the physical principles of regulation of such channels under normal and pathological conditions. In our latest projects, we supplement our biophysical methods with imaging techniques to study the role of VDAC in mitochondria during embryonic development. We also use the Seahorse real-time metabolic assay platform to investigate VDAC’s role in mitochondrial function. Among other wet-lab methods, we employ fluorescence correlation spectroscopy, bilayer overtone analysis, and confocal microscopy. Results, obtained through this rich combination of refined biophysical techniques with live-cell imaging and other cell biology techniques, inspire new strategies and pharmacological approaches to effectively correct the deviant molecular interactions associated with pathology in disease and development.
Beta-barrel channel response to high electric fields; analyzing empirical findings to distinguish between reversible denaturation and functional gating
Ion channels exhibit gating behavior, fluctuating between open and closed states, with the transmembrane voltage serving as one of the essential regulators of this process. Voltage gating is a fundamental functional aspect underlying the regulation of ion-selective, mostly alpha-helical, channels primarily found in excitable cell membranes. In contrast, another group of larger and less selective beta-barrel channels of a different origin is not directly associated with cell excitability. Remarkably, these channels can also undergo closing, or “gating”, induced by sufficiently strong electric fields. Once the field is removed, the channels reopen, preserving a memory of the gating process. This year, we explored the hypothesis that the voltage-induced closure of the beta-barrel channels can be seen as a form of reversible protein denaturation by the high electric fields applied in model membranes experiments, exceeding twenty million volts per meter for a typical 100 mV transmembrane potential difference used in experiments, rather than a manifestation of functional gating [Reference 1]. We re-examined the fundamental aspects of voltage-induced closing in beta-barrel channels, focusing on the outer membrane protein F (OmpF), which forms trimeric channels in the Gram-negative bacterium Escherichia coli. We reconstituted the OmpF trimers into planar lipid bilayers and analyzed various characteristics of the closing-opening process that support this idea, using multi- and single-channel measurements. Specifically, we considered the nearly symmetric response to voltages of both polarities, the presence of multiple closed states, the stabilization of the open conformation in channel clusters, the long-term gating memory, and the Hofmeister effects (changes of protein solubility triggered by neutral electrolyte co-solutes) in closing kinetics (Figure 1). The denaturation hypothesis may explain the slow progress in understanding the molecular mechanisms of beta-barrel channel response to the applied voltage. Indeed, in many cases, voltage gating of ion-selective channels of excitable cells has been successfully linked to the well defined structural changes between the open and closed conformations of the channel-forming protein molecule. Conversely, it is widely recognized that protein denaturation, as well as the folding into functional structures, is a highly complex process. Except for small proteins, the process evolves along various trajectories within a multi-dimensional energy landscape, often characterized by a plethora of poorly defined unfolded states and long-term memory, thus supporting the hypothesis of voltage-induced denaturation as the beta-barrel gating mechanism. Furthermore, we considered the evolutionary aspect of the phenomenon, proposing that the field-induced denaturation of membrane proteins might have served as a starting point for their development into remarkable molecular machines of eukaryotic cells such as the voltage-gated channels of nerve and muscle cells.
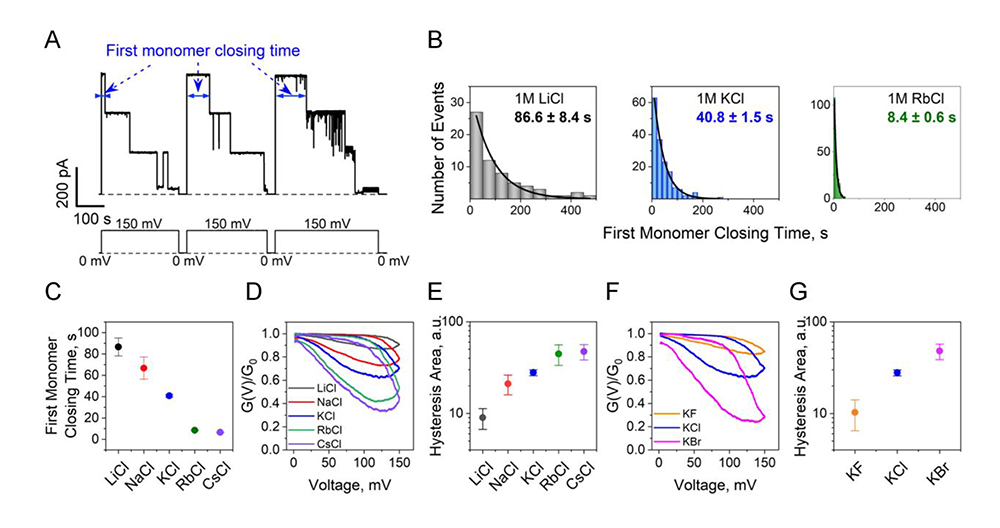
Figure 1. Hofmeister effect in OmpF voltage-induced closing
A. Examples of raw currents through a single OmpF channel at the application of 150 mV.
B. The histograms of the first monomer closing times are fitted by a single exponential function. For illustrative purposes, only the 1 M LiCl (left panel), KCl (middle panel), and RbCl (right panel) data are shown.
C. There is an order of magnitude difference in closing times in the LiCl, NaCl, KCl, RbCl, and CsCl series.
D, E. Multi-channel conductance hysteresis curves at 1 mHz ramp frequency (D) show a greater than four-fold increase in the areas encircled by hysteresis curves (E) in the LiCl, NaCl, KCl, RbCl, CsCl series.
F, G. Multichannel conductance 1 mHz hysteresis curves for different anions. The influence of anions on the OmpF voltage-induced closing and opening (F) is seen as a more than four-fold increase in the areas encircled by hysteresis curves (G) in the KF, KCl, and KBr series.
Figure 1. Hofmeister effect in OmpF voltage-induced closing
A. Examples of raw currents through a single OmpF channel at the application of 150 mV.
B. The histograms of the first monomer closing times are fitted by a single exponential function. For illustrative purposes, only the 1 M LiCl (left panel), KCl (middle panel), and RbCl (right panel) data are shown.
C. There is an order of magnitude difference in closing times in the LiCl, NaCl, KCl, RbCl, and CsCl series.
D, E. Multi-channel conductance hysteresis curves at 1 mHz ramp frequency (D) show a greater than four-fold increase in the areas encircled by hysteresis curves (E) in the LiCl, NaCl, KCl, RbCl, CsCl series.
F, G. Multichannel conductance 1 mHz hysteresis curves for different anions. The influence of anions on the OmpF voltage-induced closing and opening (F) is seen as a more than four-fold increase in the areas encircled by hysteresis curves (G) in the KF, KCl, and KBr series.
Dimeric tubulin modifies the mechanical properties of lipid bilayers; using gramicidin channel as a sensitive molecular probe.
Ion channels are known to be regulated by the mechanical properties of a lipid membrane. This year, using the gramicidin A (grA) channel as a molecular probe, we showed that binding of dimeric tubulin to planar lipid membranes changes the channel kinetics, seen as an increase in the lifetime of the grA–conducting dimer, and thus points towards modification of the membrane’s mechanical properties [Reference 4]. In addition to dimeric tubulin, we studied a synthetic peptide whose sequence corresponds to the membrane-binding helix H10 of alpha-tubulin. We showed that the effect of both compounds is more pronounced in the presence of non-lamellar lipids in the lipid mixture used for membrane formation. Specifically, in membranes containing non-lamellar DOPE (dioleoylphosphatidylethanolamine), tubulin increases grA channel lifetime, reduces channel conductance, and induces characteristic fast conductance blockages (Figure 2). In pure lamellar DOPC (dioleoylphosphatidylcholine) bilayers, the effect of tubulin on the grA channel was essentially negligible. The tubulin-induced increase of grA channel lifetime indicates that tubulin alters the membrane's lipid-packing properties upon binding to DOPE–containing membranes, thus confirming a previously suggested model. We propose that tubulin binding redistributes the lateral pressure of lipid packing along the membrane depth, making it closer to the profile expected for lamellar lipids. This redistribution happens because tubulin perturbs the lipid-headgroup spacing to reach the membrane’s hydrophobic core via its amphiphilic alpha-helical domain (Figure 2). Tubulin thus increases the forces of repulsion between the lipid headgroups and reduces such forces in the hydrophobic region. Taken together, our data suggest that tubulin-membrane binding is not defined by a specific interaction of tubulin with lipid headgroups but exhibits rather unspecific binding depending on the degree of flexibility of the headgroups of the membrane surface. We suggest that the effect is reciprocal, meaning that alterations in lipid-bilayer mechanics caused by membrane remodeling during cell proliferation in disease and development may also modulate tubulin membrane binding, thus exerting regulatory functions. One of those functions includes regulating protein-protein interactions at the membrane surface, as exemplified by VDAC complexation with tubulin. Considering that VDAC gating is known to be sensitive to lipid bilayer properties, our data suggest that, in addition to direct blocking of the VDAC pore by tubulin anionic C-terminal tails, tubulin could influence VDAC properties indirectly by modulating the packing stress of the lipid bilayer.
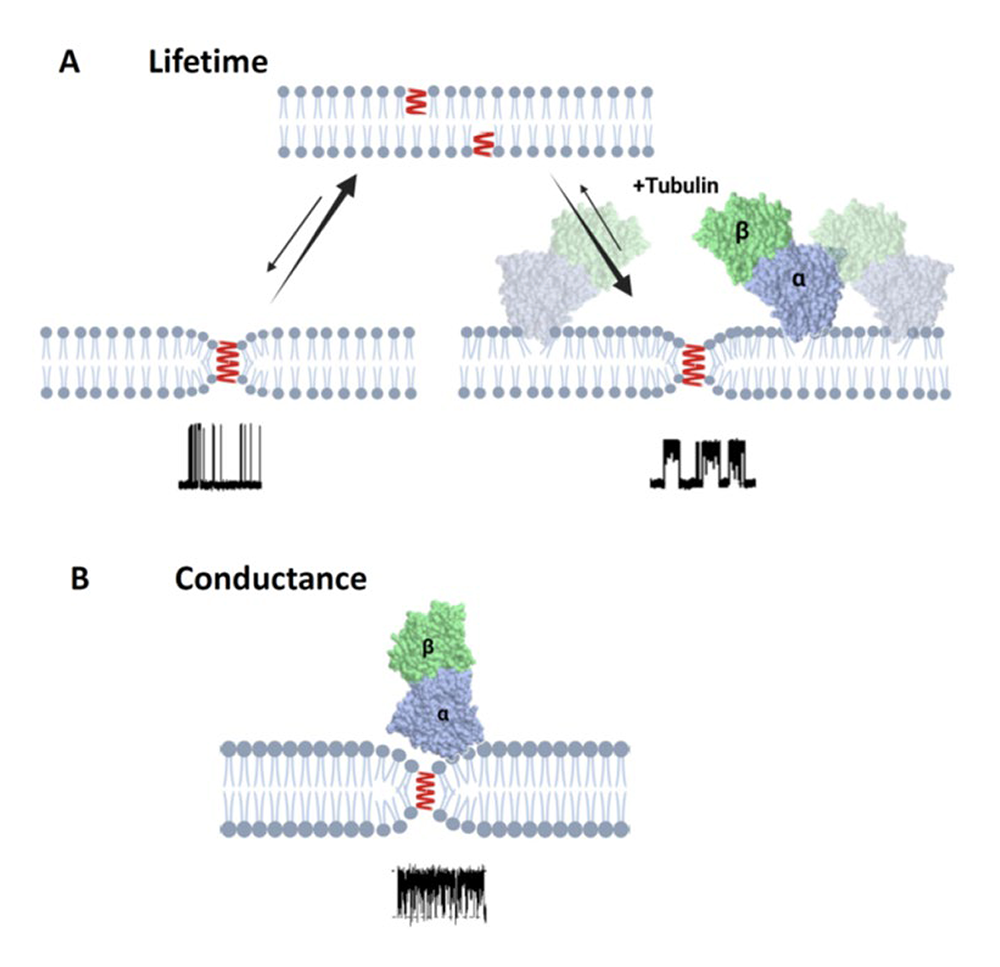
Figure 2. Schematics of the effect of tubulin dimer on grA lifetime and conductance
A. Tubulin binding to the DOPE membrane reduces packing stress of the lipid tails, which is observed as the increase of grA lifetime.
B. In the case of diC(22:1)PC membranes, binding of the tubulin dimers is limited to the regions of membranes where headgroup packing is distorted by grA channel presence in the region of the lipid funnel forming the entrance to the channel. This limitation leads to the unchanged integral properties of the membrane and unchanged grA lifetime; however, the localized binding is clearly manifested via transient channel blockages by the bulky body of the tubulin dimer. Created with Biorender.com.
Figure 2. Schematics of the effect of tubulin dimer on grA lifetime and conductance
A. Tubulin binding to the DOPE membrane reduces packing stress of the lipid tails, which is observed as the increase of grA lifetime.
B. In the case of diC(22:1)PC membranes, binding of the tubulin dimers is limited to the regions of membranes where headgroup packing is distorted by grA channel presence in the region of the lipid funnel forming the entrance to the channel. This limitation leads to the unchanged integral properties of the membrane and unchanged grA lifetime; however, the localized binding is clearly manifested via transient channel blockages by the bulky body of the tubulin dimer. Created with Biorender.com.
Solute translocation probability, lifetime, and “rectification” in membrane channels with localized constriction; theoretical analysis in the framework of a continuous diffusion model
We performed a theoretical analysis of a solute molecule's translocation probability and lifetime in a cylindrical membrane channel containing a localized constriction at an arbitrary location [Reference 5]. Membrane channels are complex protein structures characterized by significant variations in the radius of the hydrophilic pore along the channel axis. Geometries of the water-filled pores usually incorporate combinations of relatively large vestibules at their entrances with narrow constrictions within. The constrictions play the role of selectivity filters, whose dimensions are small enough to allow for strong interactions between the protein residues and the solute molecules attempting to pass through the channel. The example most relevant to our ongoing studies is that of the VDAC, the monomeric beta-barrel channel built of 19 beta-strands arranged into a large hydrophilic pore of about 2.7 nm diameter and 4.0 nm length. The pore contains a constriction formed by the protein’s N-terminus, which folds inside the pore as a broken alpha-helix lying along one side of the pore wall, approximately in the middle of the channel. This creates a localized narrowing of about 1.4 nm. This year, using a one-dimensional continuous diffusion approach to solute dynamics in the channel, we explored two models to describe the effect of a constriction. The first considers a molecule’s interaction with the constriction in terms of a narrow rectangular barrier in the potential of mean force. The second novel model proposed in our study represents this interaction by introducing an infinitely thin permeable partition. We demonstrated that, when the parameters of the two models are chosen to warrant the same translocation probability, both models predict the same mean lifetime of the molecule in the channel. While the translocation probability is independent of the constriction location, the mean lifetime is a function of its location. The benefit of the proposed model is that it allows one to combine the height and length of the potential barrier into a single parameter, which is the partition’s permeability. We showed that, in the case of an asymmetric location of the localized constriction and strong repulsion between the solutes, the solute flux through the channel is a function of the direction in which it goes (Figure 3), analogous to the phenomenon known in ion channel electrophysiology as rectification.
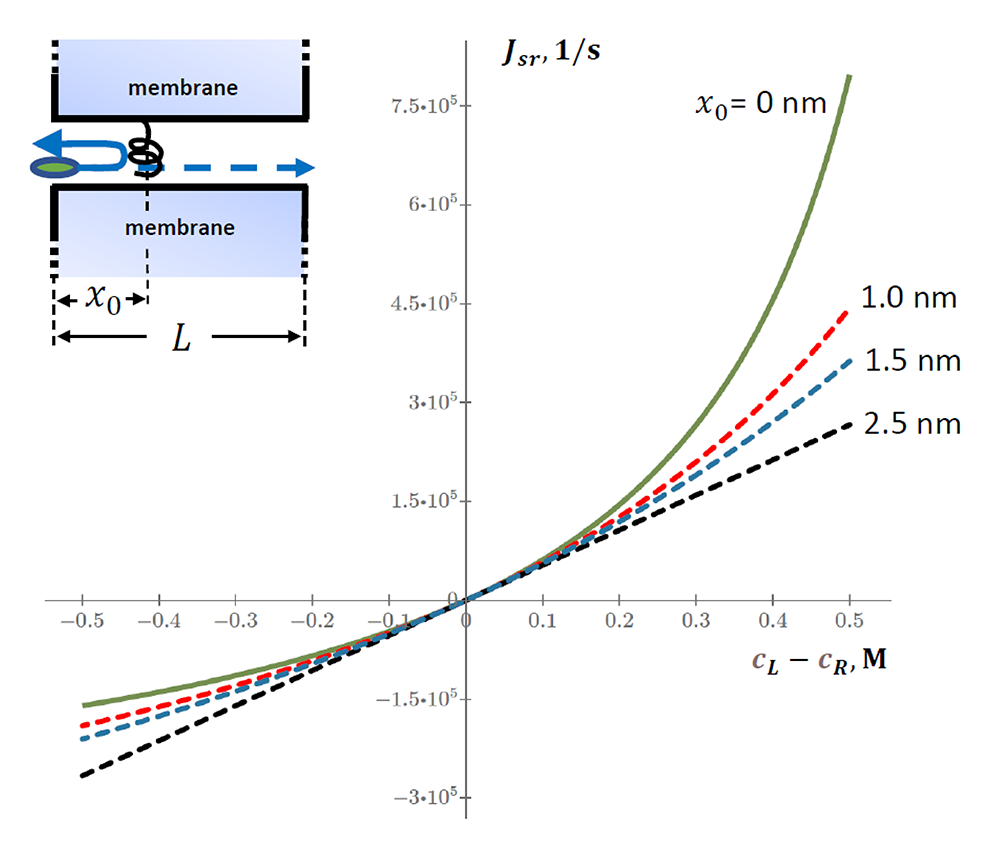
Figure 3. Flux through the channel as a function of the concentration difference at different constriction localizations
The graph shows rectification at all localizations of the constriction except for xo = L/2 = 2.5 nm (middle of the channel). Concentrations are varied in a way that keeps their sum constant at cL + cR =3.01x1026 m-3, which corresponds to 0.5 M. Other parameters are given in Reference 5.
Figure 3. Flux through the channel as a function of the concentration difference at different constriction localizations
The graph shows rectification at all localizations of the constriction except for xo = L/2 = 2.5 nm (middle of the channel). Concentrations are varied in a way that keeps their sum constant at cL + cR =3.01x1026 m-3, which corresponds to 0.5 M. Other parameters are given in Reference 5.
Additional Funding
- Graduate Partnerships Program for Antara Syam
Publications
- Beta-barrel channel response to high electric fields: Functional gating or reversible denaturation? Int J Mol Sci 2023 24:16655
- Counter-intuitive features of particle dynamics in nanopores. Int J Mol Sci 2023 24:15923
- Diffusion resistance of segmented channels. J Phys Chem B 2023 127:7291–7298
- Dimeric tubulin modifies mechanical properties of lipid bilayer as probed by gramicidin A channel. Int J Mol Sci 2024 25:2204
- Solute translocation probability, lifetime, and “rectification” in membrane channels with localized constriction. Phys Chem Chem Phys 2024 26:15758–15764
Collaborators
- Vicente M. Aguilella, PhD, Universidad Jaume I, Castellón, Spain
- Leonardo Dagdug, PhD, Universidad Autónoma Metropolitana-Iztapalapa, Mexico City, Mexico
- David Hoogerheide, PhD, National Institute of Standards and Technology, Gaithersburg, MD
- Ekaterina M. Nestorovich, PhD, The Catholic University of America, Washington, DC
- William M. Rosencrans, BS, California Institute of Technology, Pasadena, CA
- Alexander Sodt, PhD, Unit on Membrane Chemical Physics, NICHD, Bethesda, MD
- Michael Weinrich, MD, Office of the Director, NICHD, Bethesda, MD
- Joshua Zimmerberg, MD, PhD, Section on Integrative Biophysics, NICHD, Bethesda, MD
Contact
For more information, email bezrukos@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/Bezrukov.