Neuronal Circuits Controlling Behavior: Genetic Analysis in Zebrafish
- Harold Burgess,
PhD, Head, Section on Behavioral Neurogenetics - Tripti Gupta, PhD, Staff Scientist
- Deepthi Nammi, PhD, Visiting Fellow
- Madalina Robea, PhD, Visiting Fellow
- Svetlana Semenova, PhD, Visiting Fellow
- Hariom Sharma, PhD, Visiting Fellow
- Noel McGrory, BSc, Postbaccalaureate fellow
- Amir Sams, BSc, Postbaccalaureate fellow
- Reid Doctor, BSc, Graduate Student
- Jennifer L. Sinclair, MSc, Zebrafish Technician

The Section on Behavioral Neurogenetics studies the development and function of neural circuits for sensory processing, and how these are modified in neurodevelopmental disorders. We use the larval zebrafish as a model because its brain exhibits the basic architecture of the vertebrate brain but is much less complex than the mammalian brain. Despite the relative simplicity of their nervous system, zebrafish have a sophisticated repertoire of sensory-guided and internally driven behaviors. Furthermore, the optical clarity of the embryo facilitates visualization of individual neurons and their manipulation with genetic techniques. Behavior in larvae is innate and thus exhibits minimal variability between fish. Subtle alterations in behavior can therefore be robustly measured, making it possible to quickly assess the contribution of identified neurons to a variety of motor behaviors.
We focus on two aspects of behavioral regulation: the neuronal mechanisms by which sensory context regulates behavioral decisions; and the pathways that sustain changes in behavioral state. Neuronal connections that allow the brain to integrate sensory and internal-state information are established through genetic interactions during development, and are frequently disrupted by gene mutations associated with neurodevelopmental disorders. We can therefore use discoveries about sensorimotor integration pathways to understand how human disease genes disrupt brain development. To support these objectives, we develop new genetic tools and behavioral assays to probe the nexus between neuronal function and behavior at single-cell resolution.
Neuronal pathways for auditory sensory processing
Startle responses are rapid reflexes that are triggered by sudden sensory stimuli, and which help animals defend against, or escape from, potentially threatening stimuli. In both fish and mammals, startle responses are initiated by giant reticulospinal neurons in the medulla, which receive short-latency sensory input from diverse sensory modalities. Although highly stereotyped, startle responses are nevertheless modulated by sensory context and behavioral state and are therefore an excellent system in which to study how such information is integrated. Moreover, the underlying sensorimotor circuits are relatively simple, facilitating studies into the effects of gene mutations that cause neurodevelopmental disorders such as autism and schizophrenia.
In mammals, including humans, the startle response to a strong auditory stimulus can be inhibited by pre-exposure to a weak acoustic ‘prepulse,’ a form of startle modulation termed prepulse inhibition, which is diminished in several neurological conditions. Previously, we showed that, in zebrafish, as in mammals, several distinct cellular mechanisms mediate prepulse inhibition, depending on the time interval between the prepulse and the startle stimulus, with NMDA receptor signaling playing a key role for intervals greater than 100 ms. Accordingly, zebrafish with gene mutations in specific NMDA receptor subunits linked to schizophrenia show diminished prepulse inhibition. We have now also identified neurons that mediate prepulse inhibition at intervals of less than 100 ms and confirmed previous reports that a GABAergic mechanism is involved (Figure 1). In ongoing experiments, we are now defining how such neurons suppress startle reflexes by examining their synaptic connections with, and molecular signaling to, central neurons that initiate startle responses.
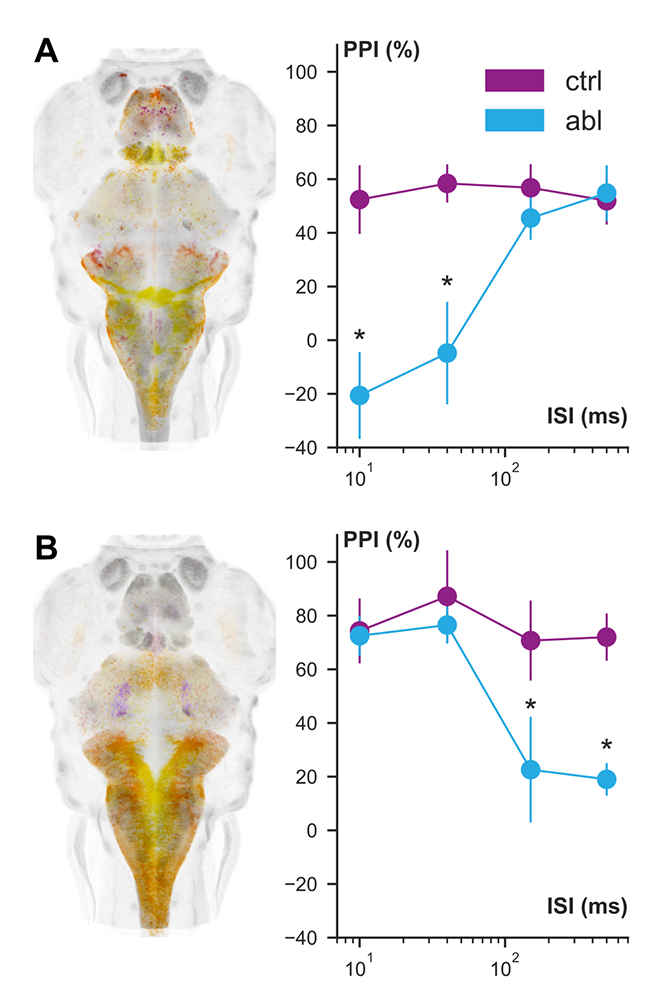
Figure 1. Identification of neurons that mediate prepulse inhibition
In prepulse inhibition (PPI), startle responses to an intense auditory stimulus are suppressed when preceded 10–1000 ms by a weak auditory cue. By screening transgenic Gal4 lines, we identified lines (left panels, depth-coded maximum projection) that label neurons required for PPI at short (10,40 ms) interstimulus intervals (A) and long (150,500 ms) interstimulus intervals (B). In these experiments, the Gal4 line indicated is used to drive a UAS:Nitroreductase line, allowing us to ablate labeled neurons.
Figure 1. Identification of neurons that mediate prepulse inhibition
In prepulse inhibition (PPI), startle responses to an intense auditory stimulus are suppressed when preceded 10–1000 ms by a weak auditory cue. By screening transgenic Gal4 lines, we identified lines (left panels, depth-coded maximum projection) that label neurons required for PPI at short (10,40 ms) interstimulus intervals (A) and long (150,500 ms) interstimulus intervals (B). In these experiments, the Gal4 line indicated is used to drive a UAS:Nitroreductase line, allowing us to ablate labeled neurons.
Our understanding of the cellular pathway that links auditory sensory cues to startle behavior has important gaps. We therefore defined afferent connectivity from the auditory sensory ganglion in zebrafish (the statoacoustic ganglion), in order to elucidate pathways that transmit behaviorally relevant vibration and acceleration signals to central regions that process sensory information for startle behavior, balance, and eye movements. Given the small size of the larvae, a challenge is to link neuronal activity to behavior. Thus, to non-invasively record neuronal activity in these experiments, we built a new microscope that allows us to record changes in transgenically expressed GCaMP fluorescence in freely swimming larvae (Figure 2).
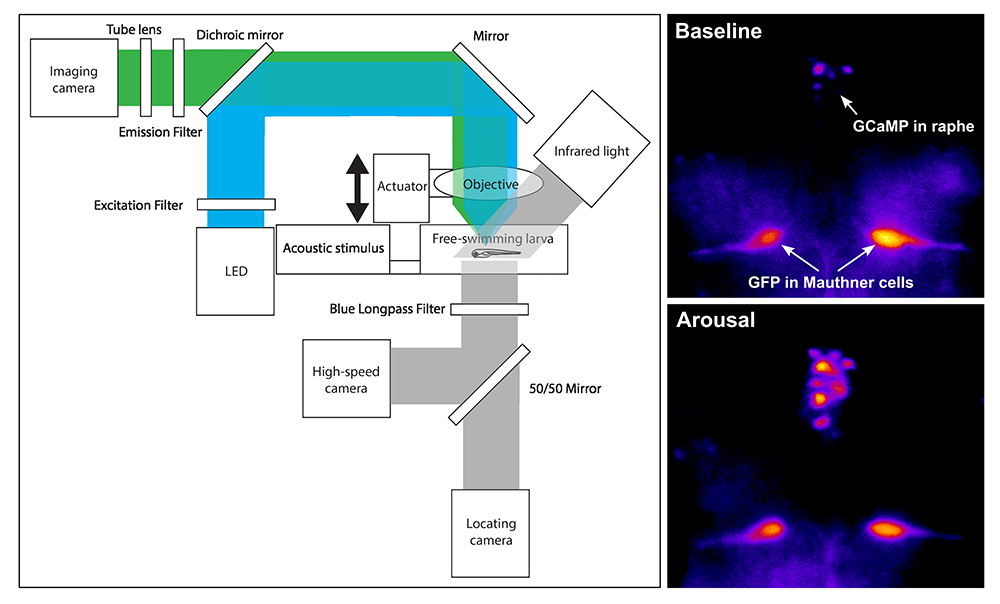
Figure 2. Development of a microscope to monitor neuronal activity in free-swimming zebrafish
To record neuronal activity in unrestrained zebrafish larvae, we developed a microscope (left panel) that uses a lens coupled to a fast actuator that can rapidly acquire a complete brain image of GCaMP fluorescence from a free-swimming larva. The system uses a high-speed camera to record behavioral responses to an auditory cue, and low-speed camera to monitor the position of the larva in the arena. As proof of principle (right panel), we show that activity in raphe neurons is low at baseline, and high during arousal. We also express GFP (green fluorescent protein) in separate neurons in samples to enable fluorescence-intensity normalization.
Figure 2. Development of a microscope to monitor neuronal activity in free-swimming zebrafish
To record neuronal activity in unrestrained zebrafish larvae, we developed a microscope (left panel) that uses a lens coupled to a fast actuator that can rapidly acquire a complete brain image of GCaMP fluorescence from a free-swimming larva. The system uses a high-speed camera to record behavioral responses to an auditory cue, and low-speed camera to monitor the position of the larva in the arena. As proof of principle (right panel), we show that activity in raphe neurons is low at baseline, and high during arousal. We also express GFP (green fluorescent protein) in separate neurons in samples to enable fluorescence-intensity normalization.
Neural mechanisms for behavioral-state control
Over the course of the day, motivational goals change in response to both internal and external cues. At any given moment, an individual’s behavioral state strongly influences decisions on how to interact with the environment. We aim to identify the neural systems that maintain short-term behavioral states and to determine how they interact with central mechanisms for sensory processing. We developed several paradigms in which the behavioral state of zebrafish is temporarily altered. Our strategy is to allow larvae to remain in the arena until their motor behavior achieves a stable state, then introduce a temporary perturbation to the environment and examine changes in behavior that persist on a time-scale of several minutes after the perturbation is removed.
One robust paradigm that we use to study behavioral-state control is flow-induced arousal. After exposure to a brief water flow stimulus, zebrafish larvae show elevated motor activity and sensory responsiveness that persists for several minutes after termination of water movement (Figure 3). We previously showed that the serotonergic raphe nucleus regulates sensory responsiveness during this state, but the neural basis for hyperactivity was not known. Using a genetically encoded reporter of calcium activity (CaMPARI2), we showed that flow-induced arousal is accompanied by a distributed increase in brain activity. To identify neurons that mediate this flow-induced swimming, we used a collection of transgenic lines to ablate different cohorts of neurons in the brain. We uncovered one such line, which labels neurons using the transcription factor Gal4, that are required for this behavior. By comparing the pattern of expression in this line with CaMPARI2 labeling, we could pinpoint specific neurons that mediate flow-induced swimming, and are now working to molecularly characterize these cells.
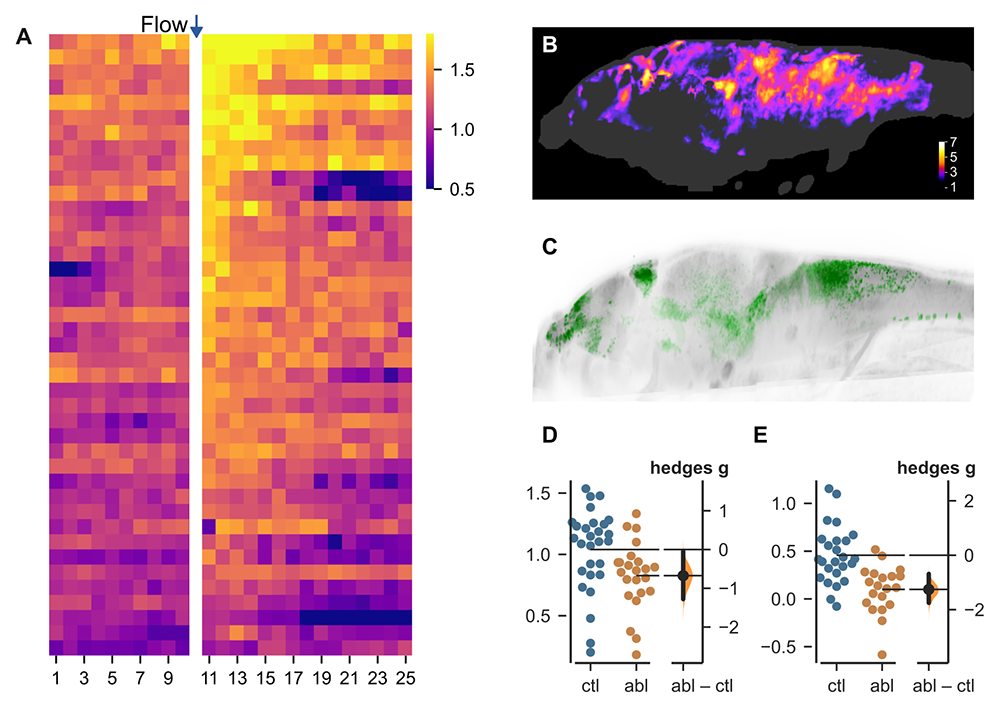
Figure 3. Neurons that mediate flow-induced swimming behavior
After exposure to water flow, larvae show persistently elevated locomotor activity for 5–10 minutes after water motion has ceased, revealing a change in internal behavioral state (heat map in A). To uncover neurons that mediate flow-induced swimming, we used fluorescent CaMPARI2 imaging to compare the pattern of neuronal activity at baseline with activity during arousal (p-value heatmap in B). We also performed an ablation screen of Gal4 transgenic lines and identified a line (C) in which ablation of the labeled neurons leads to a small reduction in baseline movement (D) but a very large reduction in flow-induced swimming (E).
Figure 3. Neurons that mediate flow-induced swimming behavior
After exposure to water flow, larvae show persistently elevated locomotor activity for 5–10 minutes after water motion has ceased, revealing a change in internal behavioral state (heat map in A). To uncover neurons that mediate flow-induced swimming, we used fluorescent CaMPARI2 imaging to compare the pattern of neuronal activity at baseline with activity during arousal (p-value heatmap in B). We also performed an ablation screen of Gal4 transgenic lines and identified a line (C) in which ablation of the labeled neurons leads to a small reduction in baseline movement (D) but a very large reduction in flow-induced swimming (E).
Zebrafish models of neurodevelopmental disorders
We collaborate widely with clinicians to generate and characterize zebrafish models for mutations discovered in humans (often through exome sequencing) that are likely to have a neurodevelopmental origin. We use the CRISPR/Cas9 system to generate lesions in zebrafish genes that are homologous to those disrupted in the human disorders. We then apply behavioral analysis, transcriptomics, and voxel-based morphometry as part of a broad phenotyping strategy. A unique feature of brain imaging in zebrafish is the ability to visualize the total architecture of the brain while simultaneously recording the position and morphology of every constituent labeled neuron. In order to compare the distribution of neurons in different larvae, we optimized a protocol that permits highly precise brain registration [Reference 1]. Such high precision of alignment permits statistically robust whole-brain analysis of neuronal composition and morphology in zebrafish mutant models, pinpointing brain regions with changes that are difficult to detect visually. The technique can be applied to almost any zebrafish neurodevelopmental model, thereby enabling robust and quantitative detection of subtle changes in brain structure or composition. Through this work, we aim to provide insight into the fundamental molecular and cellular processes associated with each disorder.
Using voxelwise morphometry, we discovered that mutations in xrcc1, a human disease gene, leads to a highly localized reduction in tissue volume in the larval cerebellum (Figure 4). Xrcc1 is a scaffold protein that negatively regulates Parp1, a protein involved in single-strand break repair. We showed that cerebellar defects in xrcc1 mutants can be rescued by simultaneously knocking down parp1, suggesting that parp1 inhibitors might be useful therapeutic candidates for xrcc1–related disorders [Reference 2].
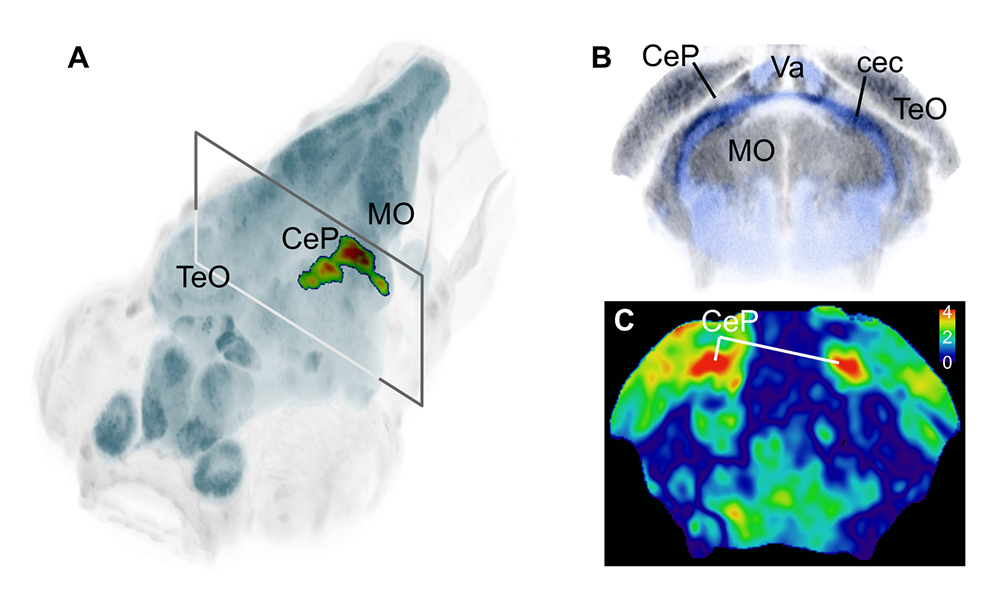
Figure 4. Loss of xrcc1 leads to a localized loss of cerebellar tissue.
Using CRISPR/Cas9, we introduced mutations into xrcc1, then performed brain-wide morphometry to assess changes in brain structure or composition. Mutants had a significant reduction in volume in part of the cerebellum, as seen in the 3D projection (A) and coronal slice at the level indicated (B–C). Color bar in C indicates –1×log10(p-value), revealing a bilateral cluster of voxels in the cerebellar plate.
Figure 4. Loss of xrcc1 leads to a localized loss of cerebellar tissue.
Using CRISPR/Cas9, we introduced mutations into xrcc1, then performed brain-wide morphometry to assess changes in brain structure or composition. Mutants had a significant reduction in volume in part of the cerebellum, as seen in the 3D projection (A) and coronal slice at the level indicated (B–C). Color bar in C indicates –1×log10(p-value), revealing a bilateral cluster of voxels in the cerebellar plate.
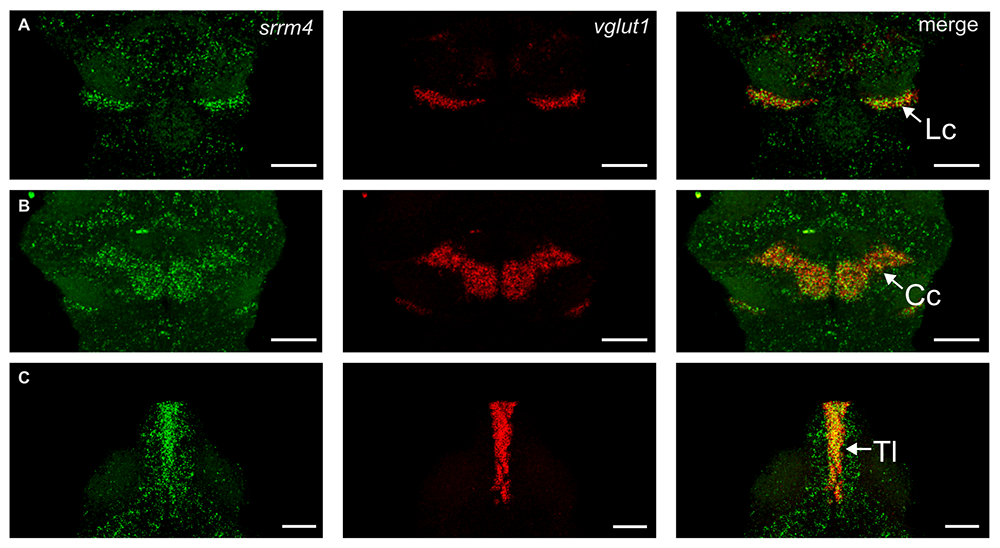
We also studied srrm4, which earlier studies had suggested encodes a key regulator of alternatively spliced microexons during neural development that were implicated in the etiology of autism. We found that srrm4 is indeed expressed during neural development in zebrafish, enriched in granule populations in the cerebellum and torus longitudinalis. However, we could not reproduce previously described morpholino phenotypes in mutants, and found that loss of srrm4 had little effect on neural development or microexon splicing in zebrafish (Figure 5) [Reference 3].
Figure 5. Srrm4 is expressed in multiple granule-cell populations in the developing brain.
In assessing expression of srrm4 during neural development in zebrafish, we noted particular expression two groups of cells in the cerebellum (green, A–B). Co-expression with vglut1 confirmed that cells enriched in srrm4 were granule cells (red). Intriguingly, we also observed strong srrm4 expression in a second granule-cell population in the torus longitudinalis (C).
Figure 5. Srrm4 is expressed in multiple granule-cell populations in the developing brain.
In assessing expression of srrm4 during neural development in zebrafish, we noted particular expression two groups of cells in the cerebellum (green, A–B). Co-expression with vglut1 confirmed that cells enriched in srrm4 were granule cells (red). Intriguingly, we also observed strong srrm4 expression in a second granule-cell population in the torus longitudinalis (C).
Publications
- Brain imaging and registration in larval zebrafish. Methods Mol Biol 2024 2707:141–153
- Parp1 deletion rescues cerebellar hypotrophy in xrcc1 mutant zebrafish. BioRxiv 2024 10.1101/2024.11.25.625242
- Mutations in the microexon splicing regulator srrm4 have minor phenotypic effects on zebrafish neural development. BioRxiv 2024 10.1101/2024.11.29.626094
Collaborators
- Edward A. Burton, MD, DPhil, FRCP, University of Pittsburgh, Pittsburgh, PA
- Keith Caldecott, PhD, University of Sussex, Brighton, United Kingdom
- Thomas E. Dever, PhD, Section on Protein Biosynthesis, NICHD, Bethesda, MD
- Andy Golden, PhD, Laboratory of Biochemistry and Genetics, NIDDK, Bethesda, MD
- Todd S. Macfarlan, PhD, Section on Mammalian Epigenome Reprogramming, NICHD, Bethesda, MD
- Reza Maroofian, PhD, University College London, London, United Kingdom
- Anne O'Donnell Luria, MD, PhD, Mendelian Genomics Research Center, Broad Institute of MIT, and Harvard University, Cambridge, MA
- Nicholas Polys, PhD, Virginia Tech, Blacksburg, VA
Contact
For more information, email haroldburgess@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/burgess.