Mechanisms and Physiological Role of Osteoclasts
- Leonid V. Chernomordik,
PhD, Head, Section on Membrane Biology - Eugenia Leikina, DVM, Senior Research Assistant
- Kamram Melikov, PhD, Staff Scientist
- Elena Zaitseva, PhD, Staff Scientist
- Jarred Whitlock, PhD, Intramural Research Training Award Fellow
- Griffin Katz, BS, Postbaccalaureate Fellow
- Amit Puthan, BS, Postbaccalaureate Fellow
- Morgan Roegner, BS, Postbaccalaureate Fellow

Diverse biological processes, in which enveloped viruses infect cells, and cells from all kingdoms of life secrete, internalize, traffic and sort integral proteins, sculpt their membranes and bring together parent genomes in sexual reproduction, share a common stage: fusion of two membranes into one. Biological membrane remodeling is tightly controlled by protein machinery, but is also dependent on the lipid composition of the membranes. Whereas each kind of protein has its own individual personality, membrane lipid bilayers have rather general properties, manifested by their resistance to disruption and bending, and by their charge. Our long-term goal is to understand how proteins fuse membrane lipid bilayers. We expect better understanding of important fusion reactions will bring about new ways of controlling them and lead to new strategies for quelling diseases involving cell invasion by enveloped viruses and defects in intracellular trafficking or intercellular fusion. Our general strategy is to combine in-depth analysis of the best characterized fusion reactions with comparative analysis of diverse, less explored fusion reactions, which can reveal new kinds of fusion proteins and clarify the generality of emerging mechanistic insights. In our recent studies, we explored the role of redox signaling in the formation of multinucleated osteoclasts in in vitro models and mechanisms underlying formation of fibrous multicellular clumps of osteoprogenitors in fibrous dysplasia.
Formation of multinucleated osteoclasts depends on an oxidized species of cell surface–associated La protein.
The integrity of our bones throughout life depends on tightly regulated coordination between the bone-forming activity of osteoblasts and the bone-resorbing activity of osteoclasts. A variety of genetic and age-related skeletal disorders are linked to a disbalance in activities of bone-forming osteoblasts and bone-resorbing osteoclast, resulting in excessive bone resorption and/or insufficient synthesis and mineralization of bone.
Osteoclasts are multinucleated cells formed by the fusion of mononucleated pre-osteoclasts, and, in most cases, cells with more nuclei (i.e., generated by a larger number of fusion events) have higher resorptive activity. Recently, we identified the La protein as a novel regulator of osteoclast fusion. La, an ubiquitous protein in eukaryotes, exists primarily as a phosphorylated, nuclear species and has essential functions in the maturation of tRNA [Reference 1]. However, we discovered that La is dephosphorylated, proteolytically cleaved, and delivered to the surface of osteoclasts during multinucleation. Surface-associated La promotes cell-cell fusion and resorptive capacity in osteoclasts. The mechanisms that trigger the radical switch in the location and function of La from the nucleus to the cell surface, and from a ubiquitous RNA chaperone to an osteoclast-specific fusion regulator, remain to be understood.
Transient, moderate increases in intracellular reactive oxygen species (ROS) levels, referred to as redox signaling or a mild oxidative stress, represent a common biological switch for proteins with multiple functions in eukaryotes and play important roles in diverse cellular differentiation processes. Intracellular ROS signaling commonly induces the formation of disulfide bonds, drives structural and oligomeric transitions, and promotes the unconventional secretion of some proteins lacking a signal peptide. Alternatively, a transition in the redox state of cysteine residues can also be triggered merely by protein trafficking changes that shifts the localization of a protein from the cytosol (typically reducing) to the extracellular environment (typically oxidizing).
Bone remodeling and, more specifically, osteoclast formation depend on ROS signaling. Differentiation of osteoclast precursors induced by the cytokine RANKL quickly generates transient ROS signaling in differentiating osteoclasts, and many bone diseases, including osteoporosis, have been linked to perturbations in ROS signaling. Recent biochemical studies demonstrated that oxidizing conditions and intracellular redox signaling elicit conformational transitions and oligomerization of the La protein and promote its nucleus-to-cytoplasm shuttling. In our recent study [Reference 2], we developed the hypothesis that La’s unique regulatory role in osteoclast multinucleation and function is controlled by an ROS switch in La trafficking and function. Using antibodies that recognize reduced vs. oxidized species of La, we found that nuclear La and cell-surface La in differentiating osteoclasts represent reduced and oxidized species of the protein, respectively. Oxidized La species at the surface of osteoclasts promote their fusion and increase multinucleation. Suppressing ROS signaling in osteoclast precursors inhibits the appearance of La in the cytoplasm and at the surface of osteoclasts and suppresses fusion during osteoclast formation. Addition of the C-terminal half of La, the region required for promoting osteoclast fusion, to the extracellular surface of osteoclasts rescues this inhibition, but only if critical La cysteine residues are available for oxidation. Our data suggest that transient ROS signaling induces a shift from reduced to oxidized La species and plays a critical role in directing the delivery of La to the surface of osteoclasts and promoting multinucleation and the subsequent resorptive function of these syncytial bone remodelers (Figure 1). Furthermore, redox transition and, more specifically, oxidation of cysteines 232 and 245 in the C-terminal half of La may represent promising targets for therapeutic strategies aimed at modulating osteoclast-dependent bone resorption in skeletal pathologies.
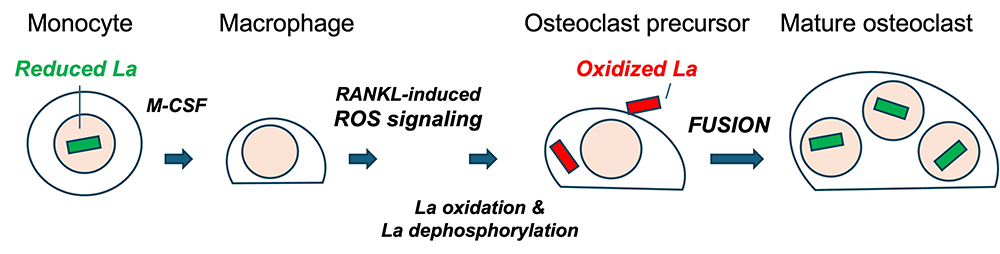
Figure 1. Formation of multinucleated osteoclasts depends on redox signaling–induced trafficking of La protein to the surface of osteoclast precursors.
Reactive oxygen species (ROS) signaling–induced restructuring of La from a reduced to an oxidized species triggers La re-localization from nucleus to the surface of differentiating osteoclasts and promotes their fusion and resorptive function. Macrophage precursors are derived via the macrophage colony-stimulating factor (M-CSF)–activation of circulating monocytes. Osteoclast differentiation is initiated by subsequent application of the receptor activator of NF-kappaB ligand (RANKL), which elicits intracellular ROS production, leading to drastic changes in the redox state and localization of La. La transitions from a predominantly nuclear, reduced species of La in monocytes and macrophages to an oxidized, dephosphorylated species that traffics to and associates with the surface of fusion-competent osteoclasts. When an osteoclast achieves an appropriate size and fusion stops, the mature bone-resorbing multinucleated osteoclasts exhibit a predominately nuclear, reduced La species, as is typical of other eukaryotic cells [References 1 and 2].
Figure 1. Formation of multinucleated osteoclasts depends on redox signaling–induced trafficking of La protein to the surface of osteoclast precursors.
Reactive oxygen species (ROS) signaling–induced restructuring of La from a reduced to an oxidized species triggers La re-localization from nucleus to the surface of differentiating osteoclasts and promotes their fusion and resorptive function. Macrophage precursors are derived via the macrophage colony-stimulating factor (M-CSF)–activation of circulating monocytes. Osteoclast differentiation is initiated by subsequent application of the receptor activator of NF-kappaB ligand (RANKL), which elicits intracellular ROS production, leading to drastic changes in the redox state and localization of La. La transitions from a predominantly nuclear, reduced species of La in monocytes and macrophages to an oxidized, dephosphorylated species that traffics to and associates with the surface of fusion-competent osteoclasts. When an osteoclast achieves an appropriate size and fusion stops, the mature bone-resorbing multinucleated osteoclasts exhibit a predominately nuclear, reduced La species, as is typical of other eukaryotic cells [References 1 and 2].
Ex vivo model of fibrous dysplasia: pre-osteoblast proliferation in fibrous lesions depends on osteoclast signaling.
Fibrous dysplasia (FD) is a benign, pediatric, disabling skeletal disease for which there are no established treatments. FD is caused by somatic, mosaic gain-of-function mutations in GNAS (the gene that encodes the stimulatory G-protein alpha subunit Gαs) within osteoblast lineage cells. Growing evidence supports inhibiting the osteoclastogenic factor receptor activator of nuclear kappa-B ligand (RANKL) as a potential treatment strategy. In this study [Reference 3], we investigated the mechanisms underlying RANKL inhibition in FD tissue and its likely indirect effects on osteoprogenitors by evaluating human FD tissue pre- and post-treatment in a phase 2 clinical trial of the anti-RANKL antibody denosumab (NCT03571191), as well as in an doxycycline-inducible murine model of FD and in ex vivo preclinical models. Histological analysis of human and mouse tissue demonstrated increased osteogenic maturation, reduced cellularity, and reduced expression of the pathogenic Gαs variant in FD lesions after RANKL inhibition. The interaction between osteoclasts and mutant osteoprogenitors was further assessed in our recently developed ex vivo lesion model [Reference 4], using whole bone marrow from un-induced FD mice and containing adherent cells of the monocytic lineage and bone marrow stem cells (BMSC), which are progenitors of osteoclasts and osteoblasts/osteocytes respectively. The addition of doxycycline to the culture media induced expression of the GαsR201C cassette (a common gain-of-function mutation in osteoprogenitors that leads to the excessive production of RANKL and the ectopic osteoclast formation that underpins the pathophysiology of FD) only in BMSCs. As expected in FD, in our ex vivo model, doxycycline robustly induced osteoprogenitor proliferation and formation of fibrous multicellular clumps, characteristic for this disease. The question arises as to whether formation of these clumps of pre-osteoblasts is a direct result of expression of mutant GNAS in these cells or whether it depends on osteoclast-to-osteoblast signaling.
After induction, BMSCs released osteoclastogenic factors, leading to the fusion of mononuclear precursors into TRAP+ (tartrate-resistant acid phosphatase, a histochemical marker of osteoclasts) multinucleated osteoclasts (Figure 2). Multinucleated osteoclasts were no longer observed after treating FD cultures with anti–RANKL antibody. We hypothesized that fibrous multicellular clumps are formed by proliferating pre-osteoblasts. If osteoclast formation is a pre-requisite for pre-osteoblast proliferation, we expect it to be inhibited by suppressing osteoclastogenic differentiation. Indeed, we found that anti–RANKL antibodies block increased proliferation of the doxycycline-induced osteoprogenitor, uncovering the important role of osteoclasts in the proliferation of abnormal FD osteoprogenitors. The results of this study demonstrated that, in addition to its expected anti-osteoclastic effect, the anti-RANKL antibody denosumab reduces FD lesion activity by decreasing FD cell proliferation and increasing osteogenic maturation, leading to increased bone formation within lesions. Our data suggest that osteoclasts contribute to the proliferation of abnormal FD osteoprogenitors, possibly through the release of extracellular vesicles (EVs) carrying TGS101 (found in EVs regardless of the type of cell from which they originate) and RANK (marker of osteoclast EVs). These findings: (1) explain why osteoclast-targeting reagents suppress the development of fibrous, multicellular lesions formed by proliferation of pre-osteoblasts; (2) highlight the unappreciated role of osteoclast-to-pre-osteoblast crosstalk as a driver of fibrous dysplasia pathology; and (3) demonstrate a novel mechanism of action of denosumab in the treatment of bone disease. We propose that the mechanisms by which osteoclasts regulate osteoblast proliferation in FD can be also at work in life-long remodeling of human skeleton.
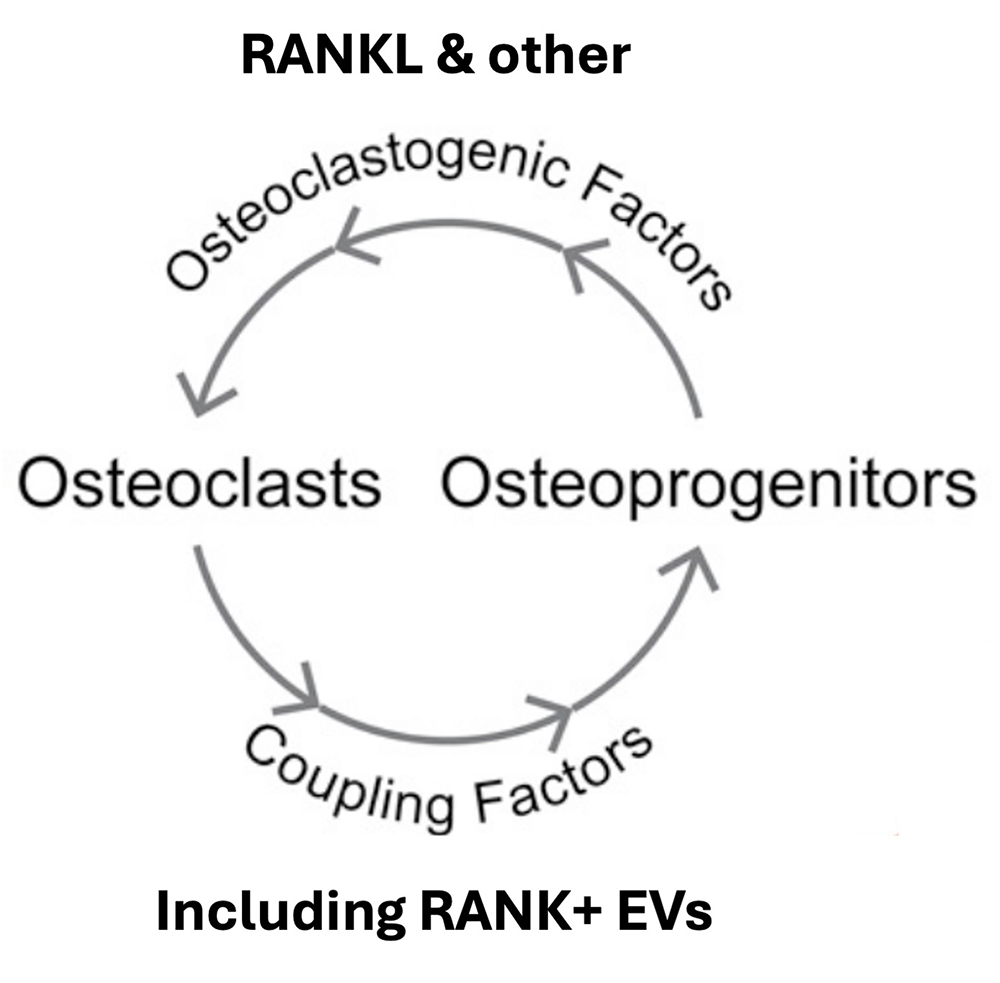
Figure 2. Osteoclast to pre-osteoblast signaling in fibrous dysplasia
After induction of a fibrous dysplasia phenotype, the cells of osteoblast lineage release RANKL and other osteoclastogenic factors promoting excessive osteoclast formation. In their turn, osteoclasts release coupling factors, including RANK–carrying extracellular vesicles, which promote excessive proliferation of pre-osteoblasts and the formation of fibrous multicellular clumps. The dependence of pre-osteoblast proliferation on osteoclast-to-osteoblast signaling explains why osteoclast-targeting reagents, such as anti-RANKL antibody, suppress the development of fibrous lesions that are characteristic of fibrous dysplasia [References 3 and 4].
Figure 2. Osteoclast to pre-osteoblast signaling in fibrous dysplasia
After induction of a fibrous dysplasia phenotype, the cells of osteoblast lineage release RANKL and other osteoclastogenic factors promoting excessive osteoclast formation. In their turn, osteoclasts release coupling factors, including RANK–carrying extracellular vesicles, which promote excessive proliferation of pre-osteoblasts and the formation of fibrous multicellular clumps. The dependence of pre-osteoblast proliferation on osteoclast-to-osteoblast signaling explains why osteoclast-targeting reagents, such as anti-RANKL antibody, suppress the development of fibrous lesions that are characteristic of fibrous dysplasia [References 3 and 4].
Additional Funding
- Binational Science Foundation (BSF) Award 2021168 (2023–2026)
Publications
- Cell surface-bound La protein regulates the cell fusion stage of osteoclastogenesis. Nat Commun 2023 14:616
- Formation of multinucleated osteoclasts depends on an oxidized species of cell surface-associated La protein. Elife 2024 13:RP98665
- RANKL inhibition reduces lesional cellularity and Gαs variant expression and enables osteogenic maturation in fibrous dysplasia. Bone Res 2024 12(1):10
- An inducible explant model of osteoclast-osteoprogenitor coordination in exacerbated osteoclastogenesis. iScience 2023 26:106470
Collaborators
- Alison Boyce, MD, Metabolic Bone Disorders Unit, NIDCR, Bethesda, MD
- Michael Collins, MD, Skeletal Disorders & Mineral Homeostasis Section, NIDCR, Bethesda, MD
- Michael M. Kozlov, PhD, DHabil, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- Leonid Margolis, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
Contact
For more information, email chernoml@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/chernomordik.