Chromatin Remodeling and Gene Activation
- David J. Clark,
PhD, Head, Section on Chromatin and Gene Expression - Peter Eriksson, PhD, Staff Scientist
- Hemant K. Prajapati, PhD, Research Fellow
- Lalita Panigrahi, PhD, Visiting Fellow
- Zhuwei Xu, PhD, Visiting Fellow
- Kenneth Wu, PhD, Postdoctoral Intramural Research Training Award Fellow

Aberrant gene regulation is the basis of many disease states. Our main objective is to understand how genes are activated for transcription in the context of chromatin structure. Chromatin is not just a packaging system for DNA in eukaryotic cells; it also participates in gene regulation. It is thought that gene regulation involves either attenuation of the inherently repressive properties of nucleosomes to facilitate gene expression, or enhancement of those properties to ensure complete repression. These events are choreographed by DNA sequence–specific transcription factors (activators and repressors) and chromatin-remodeling complexes. The latter can be divided into two groups: histone- or DNA–modifying enzymes that implement the “epigenetic code,” and ATP–dependent remodeling machines that move or displace nucleosomes. Our studies focus primarily on the roles of the ATP–dependent chromatin remodelers in gene regulation.
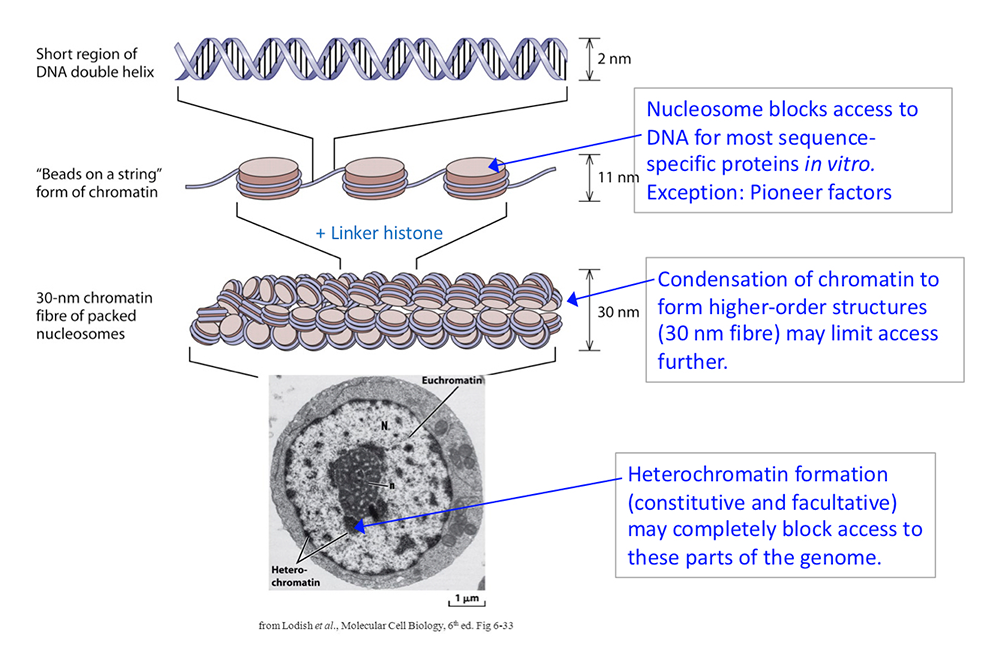
The packaging of chromosomal DNA in eukaryotic nuclei occurs at several structural levels (Figure 1). The basal structure resembles beads-on-a-string, in which the beads are nucleosome cores and the string is connecting linker DNA. The nucleosome core is composed of about 147 bp of DNA wrapped in 1.7 negative super-helical turns around a central histone octamer containing two molecules each of the four core histones (H2A, H2B, H3, and H4). The nucleosomal filament coils spontaneously into a heterogeneous higher-order approximately 30 nm–wide structure, facilitated by linker histones. The 30 nm nucleosomal filament is organized into loop domains by other chromatin proteins, such as condensin and the transcriptional regulator CTCF. Inactive chromatin (heterochromatin) is generally much more condensed than active chromatin (euchromatin) and is associated with specific proteins such as heterochromatin protein 1 (HP1) and Polycomb repressor complexes.
Figure 1. DNA packaging in the nucleus: to what extent does chromatin compaction limit access to the DNA?
DNA is packaged into the nucleus by histones. The basic structural subunit of chromatin is the nucleosome core, which contains about 147 bp of DNA wrapped nearly twice around a central octamer of core histones. Nucleosomes are regularly spaced along the DNA like beads on a string; the intervening DNA is called the linker DNA and is bound by linker histone (H1). The beads-on-a-string fiber spontaneously condenses into a heterogeneous fiber of about 30 nm width. Genomic regions rich in repetitive elements form constitutive heterochromatin in all cells, in which the chromatin fiber is even more condensed. Facultative heterochromatin is formed on genes that should be permanently silent in a specific differentiated cell type. Heterochromatin is densely packed and darkly staining in the electron micrograph shown here. Euchromatin is less condensed (light staining) and contains active genes. We are interested in determining to what extent chromatin limits DNA accessibility. Figure adapted from Chereji et al. Genome Res 2019;29:1985–1995.
Figure 1. DNA packaging in the nucleus: to what extent does chromatin compaction limit access to the DNA?
DNA is packaged into the nucleus by histones. The basic structural subunit of chromatin is the nucleosome core, which contains about 147 bp of DNA wrapped nearly twice around a central octamer of core histones. Nucleosomes are regularly spaced along the DNA like beads on a string; the intervening DNA is called the linker DNA and is bound by linker histone (H1). The beads-on-a-string fiber spontaneously condenses into a heterogeneous fiber of about 30 nm width. Genomic regions rich in repetitive elements form constitutive heterochromatin in all cells, in which the chromatin fiber is even more condensed. Facultative heterochromatin is formed on genes that should be permanently silent in a specific differentiated cell type. Heterochromatin is densely packed and darkly staining in the electron micrograph shown here. Euchromatin is less condensed (light staining) and contains active genes. We are interested in determining to what extent chromatin limits DNA accessibility. Figure adapted from Chereji et al. Genome Res 2019;29:1985–1995.
The packaging of the genome apparently presents a formidable obstacle to sequence-specific transcription factors searching for their specific binding sites. Most of these factors bind very weakly, if at all, to a cognate site within a nucleosome, unless the site is situated just inside the nucleosome, where the histone–DNA contacts are weakest. However, there are some transcription factors, termed “pioneer” factors, that have evolved to bind to nucleosomal sites with high affinity. Many pioneer factors are critical for development, given that they have the potential to invade chromatin.
In vitro studies demonstrating that nucleosomes inhibit transcription-factor binding resulted in the discovery of the ATP–dependent chromatin-remodeling enzymes, which facilitate access to the DNA. These enzymes can slide nucleosomes along DNA, move the histone octamer from one DNA molecule to another, and drive nucleosomal conformational changes. Examples include the yeast SWI/SNF, RSC, ISW1, and CHD1 multi-subunit complexes, and their metazoan counterparts, including human BAF and PBAF, SNF2H/L, and CHD complexes. The enzymes play a critical role in gene regulation. They act at promoters and other regulatory elements to facilitate nucleosome removal, allowing transcription factors to bind and permitting subsequent recruitment of RNA polymerase II. Mutations in genes encoding chromatin-remodeler subunits are associated with various human diseases, including many cancers (e.g., pediatric malignant rhabdoid tumors), and autism spectrum disorder.
Genome-wide studies of nuclei from various organisms have revealed a link between the nucleosome occupancy of a promoter and gene activity: active promoters tend to be nucleosome-depleted, whereas inactive promoters are occupied by nucleosomes. This observation supports the generally accepted model that chromatin blocks access to DNA, particularly of regulatory elements such as promoters and enhancers, preventing transcription factors from binding to their cognate sites and activating transcription. Underlying this model is the premise that chromatin blocks access to the DNA. Although there is strong evidence supporting this model, it is almost all derived from studies with isolated nuclei. However, preparation of nuclei results in the loss of critical co-factors (e.g., ATP). We measured DNA accessibility genome-wide in yeast and human cell lines, both in nuclei and in living cells. We discovered that the genome is mostly inaccessible in nuclei, as expected. However, in living cells, the genome is globally accessible.
Measuring genome accessibility in living cells
We compared the accessibility of the genome in isolated nuclei and in living yeast cells using DNA methylases. We showed that methylation by the Dam DNA methylase (which methylates A in the sequence GATC) is blocked by nucleosomes in isolated nuclei. However, expression of Dam in living cells using an inducible promoter results in methylation of the entire genome, with minimal interference from nucleosomes. Using a different DNA methylase, M.SssI (which methylates C in CG), together with nanopore long-read sequencing, we showed that centromeric nucleosomes, unlike canonical nucleosomes, are exceptionally stable, protecting their DNA from methylation in vivo. The silenced mating-type loci are also inaccessible. By using a degron approach to detect nucleosome movements in living cells as remodelers are depleted, we showed that at least three ATP–dependent chromatin remodelers (RSC, ISW1, and CHD1) contribute to nucleosome dynamics in vivo.
Our data demonstrate that nucleosomes are in a continuous state of flux in living cells, but static in nuclei, presumably as a result of the loss of critical factors during isolation. The flux may involve nucleosome sliding, nucleosome removal and replacement, and/or nucleosome conformational changes, catalyzed by ATP–dependent chromatin remodelers (Figure 2). We propose that the various remodelers compete with one another in vivo, continually moving nucleosomes to different positions, resulting in a nucleosome flux that renders the yeast genome essentially transparent to transcription factors and other DNA–binding proteins. Our observations have profound implications for the chromatin field, requiring a re-examination of the roles of the chromatin remodelers in gene regulation, and of the extent to which packaging the genome into nucleosomes is actually repressive in vivo [Reference 1].
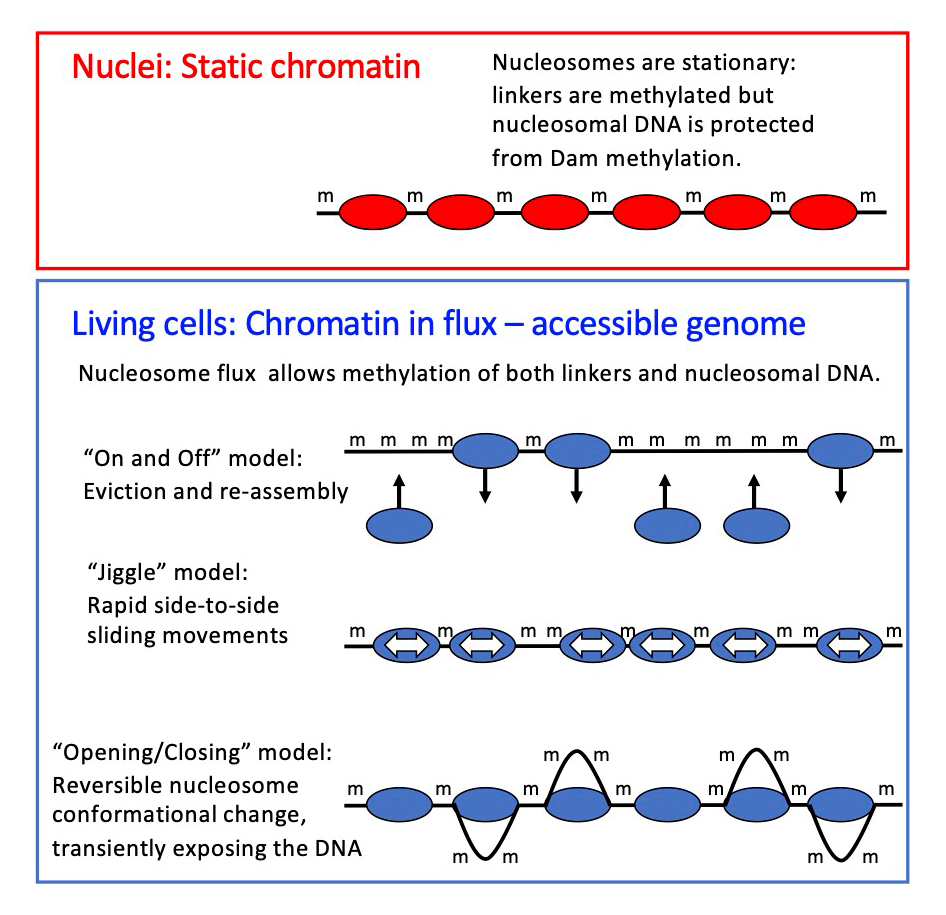
Figure 2. Chromatin flux model to explain the difference in genome accessibility in nuclei and living cells
We propose that nucleosomes are stationary in nuclei, protecting the DNA they contain (top panel). The Dam DNA methyltransferase only methylates the short stretches of linker DNA between regularly spaced nucleosomes. However, in living cells, Dam methylates virtually the entire genome (bottom panel), because the nucleosomes are in flux. Nucleosomes may be continuously removed and replaced, or shunted from side to side, or opened up, as shown, resulting in DNA exposure and methylation. We propose that the flux is generated by the competing activities of the various ATP–dependent chromatin remodelers (such as RSC, SWI/SNF, ISW1, and CHD1).
Figure 2. Chromatin flux model to explain the difference in genome accessibility in nuclei and living cells
We propose that nucleosomes are stationary in nuclei, protecting the DNA they contain (top panel). The Dam DNA methyltransferase only methylates the short stretches of linker DNA between regularly spaced nucleosomes. However, in living cells, Dam methylates virtually the entire genome (bottom panel), because the nucleosomes are in flux. Nucleosomes may be continuously removed and replaced, or shunted from side to side, or opened up, as shown, resulting in DNA exposure and methylation. We propose that the flux is generated by the competing activities of the various ATP–dependent chromatin remodelers (such as RSC, SWI/SNF, ISW1, and CHD1).
Budding yeast does not have the heterochromatin typical of the cells of higher organisms. Heterochromatin is generally associated with a high level of chromatin condensation and gene repression; it is expected to be much less accessible than transcriptionally active euchromatin. To address this important question, we are currently extending our genome accessibility studies to human cell lines.
Long-read sequencing methods, namely the PacBio and Nanopore platforms, are becoming increasingly popular, as the power of these new technologies is fully appreciated. In our own field of chromatin biology, epigenetic modifications such as 5-methylcytosine (m5C) and N6-methyladenosine (m6A) can now be detected directly in original (i.e., unamplified) multi-kilobase DNA molecules. A recent extension of this approach is the use of methylation footprinting, in which an exogenous DNA methylase is added to nuclei or chromatin, where it methylates accessible regions, such as nucleosome-depleted promoters and the linkers between nucleosomes, but not nucleosomal DNA, which it cannot access. The result is a methylation map of each molecule, to be interpreted in terms of footprints and accessible regions.
We performed methylation footprinting in budding yeast nuclei using M.EcoGII, an adenine-specific DNA methylase (Figure 3). The potential footprint resolution of this approach is very high because of the adenine density of DNA. We used PacBio long-read sequencing to detect m6A introduced by M.EcoGII, and we developed a pipeline to analyze the data. We discovered a number of critical, previously unreported, issues concerning PacBio methylation footprinting data, which must be corrected before an accurate chromatin map can be obtained. Specifically, we observed low-limit methylation levels in the genomic DNA–positive controls, a wide range in the fraction of m6A from one DNA molecule to the next, and a strong local bias against methylation of AT–rich sequences and poly(A) runs. We also found that there is a high probability of observing a single m6A base within a nucleosome, breaking up the expected roughly 147 bp footprint. These issues are primarily the result of inefficient detection of m6A by PacBio sequencing. Our novel probability model resolves all these critical interpretative problems. The pipeline output includes an IGV (Integrative Genomics Viewer)–ready bam (Binary Alignment Map) file, which displays both called m6A bases and interpretation of the methylation pattern as accessible regions and nucleosomes in individual DNA molecules.
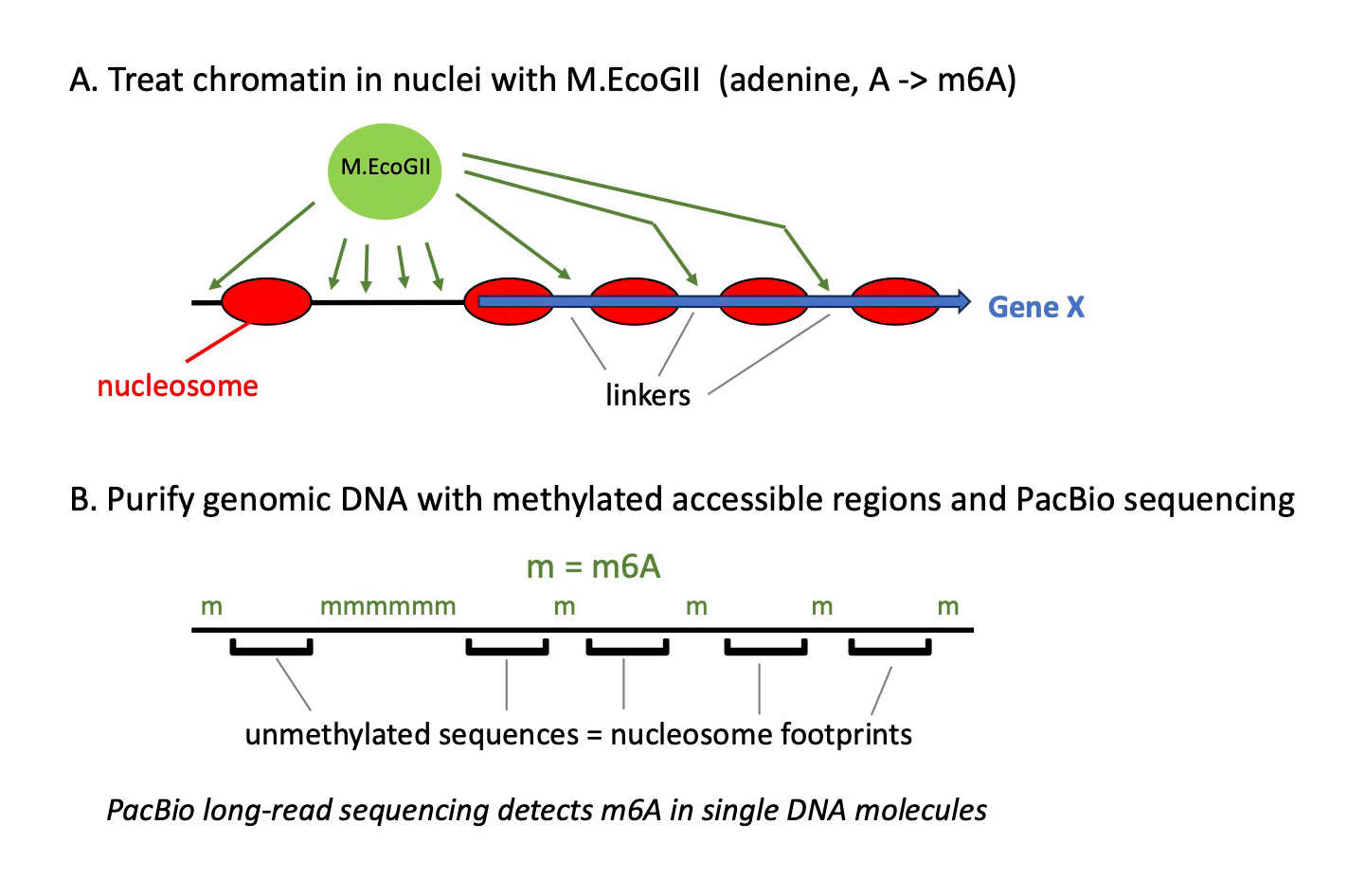
Figure 3. Mapping nucleosomes on single DNA molecules using methylation footprinting and PacBio long-read sequencing
The M.EcoGII DNA methyltransferase methylates adenine bases in DNA, converting them into 6-methyladenine (m6A).
A. Nuclei are incubated with M.EcoGII, which methylates adenines in accessible DNA, including nucleosome-depleted regions at gene promoters and the short linkers between nucleosomes. Nucleosomal DNA is protected from methylation by the histones.
B. After purification, genomic DNA is fragmented into molecules of several thousand base pairs, and sequenced using PacBio technology; m6A can be detected because the PacBio–sequencing polymerase pauses when it encounters m6A in the template strand. The result is a methylation map corresponding to accessible regions, which can be interpreted in terms of nucleosome footprints. Each mapped DNA molecule represents a stretch of DNA from a single cell. By comparing multiple molecules containing the same sequence, we detected extensive cell-to-cell variation in nucleosome positioning.
Figure 3. Mapping nucleosomes on single DNA molecules using methylation footprinting and PacBio long-read sequencing
The M.EcoGII DNA methyltransferase methylates adenine bases in DNA, converting them into 6-methyladenine (m6A).
A. Nuclei are incubated with M.EcoGII, which methylates adenines in accessible DNA, including nucleosome-depleted regions at gene promoters and the short linkers between nucleosomes. Nucleosomal DNA is protected from methylation by the histones.
B. After purification, genomic DNA is fragmented into molecules of several thousand base pairs, and sequenced using PacBio technology; m6A can be detected because the PacBio–sequencing polymerase pauses when it encounters m6A in the template strand. The result is a methylation map corresponding to accessible regions, which can be interpreted in terms of nucleosome footprints. Each mapped DNA molecule represents a stretch of DNA from a single cell. By comparing multiple molecules containing the same sequence, we detected extensive cell-to-cell variation in nucleosome positioning.
We used our data to investigate heterogeneity in chromatin structure around the transcription start sites of yeast genes. We found that nucleosome positioning on a specific gene varies widely from cell to cell, with only a small fraction of genes showing a similar, ordered nucleosomal array in every cell, even though a cell-population average is as expected from other techniques, such as MNase-Seq. We quantified the degree of heterogeneity for every yeast gene using a novel correlation score [Reference 2]. We propose that the observed heterogeneity reflects nucleosome dynamics in vivo [Reference 1], which is halted when the nuclei are isolated.
Publications
- The yeast genome is globally accessible in living cells. Nat Struct Mol Biol 2024 online ahead of print
- Examining chromatin heterogeneity through PacBio long-read sequencing of M.EcoGII methylated genomes: an m6A detection efficiency and calling bias correcting pipeline. Nucleic Acids Res 2024 52(9):e45
Collaborators
- David Levens, MD, PhD, Laboratory of Pathology, Center for Cancer Research, NCI, Bethesda, MD
- Karel Pacak, MD, PhD, DSc, Section on Medical Neuroendocrinology, NICHD, Bethesda, MD
Contact
For more information, email clarkda@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/clark.