Translational Biophotonics in Developmental Disorders and Diseases
- Amir H. Gandjbakhche,
PhD, Head, Section on Translational Biophotonics - Thien Nguyen, PhD, Research Fellow
- Jinho Park, PhD, Postdoctoral Fellow
- Soongho Park, PhD, Postdoctoral Fellow
- Wan-Chun Su, PhD, Postdoctoral Fellow
- Marc Bornstein, PhD, Special Volunteer
- Kosar Khaksari, PhD, Special Volunteer
- Tony M. George, BS, Postbaccalaureate Fellow
- Otiti Mayo, Postbaccalaureate Fellow
- John Mutersbaugh, BS, Postbaccalaureate Fellow
- Asma Sodager, Postbaccalaureate Fellow
- Wei Lun Huang, MS, Intramural Research Training Award Student
- Minghao Xue, BS, Intramural Research Training Award Student

Brain imaging and spectroscopy of developmental disorders
We used functional near-infrared spectroscopy (fNIRS) to investigate cortical activation in two distinct cohorts: (1) healthy adults and (2) infants and children, both with and without developmental disorders. The primary goal for the first cohort was to validate fNIRS findings by comparing them with results from previous fMRI and EEG studies. In the second cohort, we aimed to comprehensively explore typical and atypical developmental trajectories in infants and children, including those at elevated risk for developmental disorders. Such research efforts will help us validate fNIRS findings, provide more accurate insights into neural mechanisms through multi-modal imaging, and identify potential neuro-biomarkers for children with developmental disabilities.
Before conducting studies involving fNIRS data collection, we assessed contemporary fNIRS findings related to tracking neurodevelopmental trajectories in infants and children with and without developmental disorders. We published the results of this review [Su et al., Front Psychiatry 2023;14:121000]. Furthermore, our collaboration with Audrey Thurm continued, focusing on investigating the developmental trajectory of the mirror neuron network (MNN) from infancy to adulthood. Our exploration centers on the MNN's intricate connections with the emergence of sophisticated social behaviors in typically developing infants and those at elevated risk for developmental disorders. In an adult population, we examined the MNN during action execution and observation [Dashtestani et al. Sci Rep 2022;12:68782022]. The significance of this exploration lies in the understanding that motor-cognitive abilities are shaped and enhanced through mental rehearsals of action. Our findings highlighted the important roles of the left parietal inferior region during the imagination condition. Additionally, other brain regions within the left hemisphere were active during the motor-imagery task, mirroring the patterns observed during execution and observation conditions. Although the literature on action imagination is relatively limited, our findings align with existing studies, supporting the notion that both action observation and imagination engage the same sensory-motor cortical network that underpins the execution of the corresponding action. A plausible explanation for these consistent brain-activity patterns across the three conditions is the internal rehearsal of action, reinforcing the intrinsic connection between observation, imagination, and execution. We recently published these significant insights [Su et al. Sci Rep 2023;13:5151]. Our goal in the coming years is to recruit infant subjects and apply the action-observation-execution paradigms we developed for adults to investigate the developing MNN. By longitudinally tracking the developmental trajectories of behaviors and associated neural activity, we seek to identify potential neuro-biomarkers in children with and without developmental disorders. These research endeavors will have significant clinical implications, particularly for early identification and intervention outcome measurements.
In addition to exploration of the MNN, we applied multi-modal, simultaneous fNIRS and EEG recording in a dual-task paradigm to assess the effects of acute physical activity on neural activity during dual tasking. We recruited 18 healthy volunteers and recorded cortical activation/neural activity during tasks involving varying posture-control challenges (e.g., sitting, standing, and tandem stance) and cognitive loads (no task, congruent, and incongruent conditions during the Flanker task, a test of selective attention and inhibitory function). The results showed increased prefrontal cortex and pre- and post-central gyrus activation as the posture control challenges and cognitive loads increased during easier conditions (sitting, no task, and congruent conditions). Conversely, in more challenging conditions (tandem and incongruent conditions) the trend was the opposite. Acute physical activity significantly enhanced inhibitory control, as indicated by reduced reaction times during the Flanker task, and triggered a shift from an under- to an over-addictive cortical activation pattern. These findings support the Capacity Sharing Theory and advocate the use of acute physical activity to enhance the overall capacity for posture-inhibitory control dual-tasking. In real-world scenarios, acute physical activity could serve to boost arousal and enhance safety during dual-task performance.
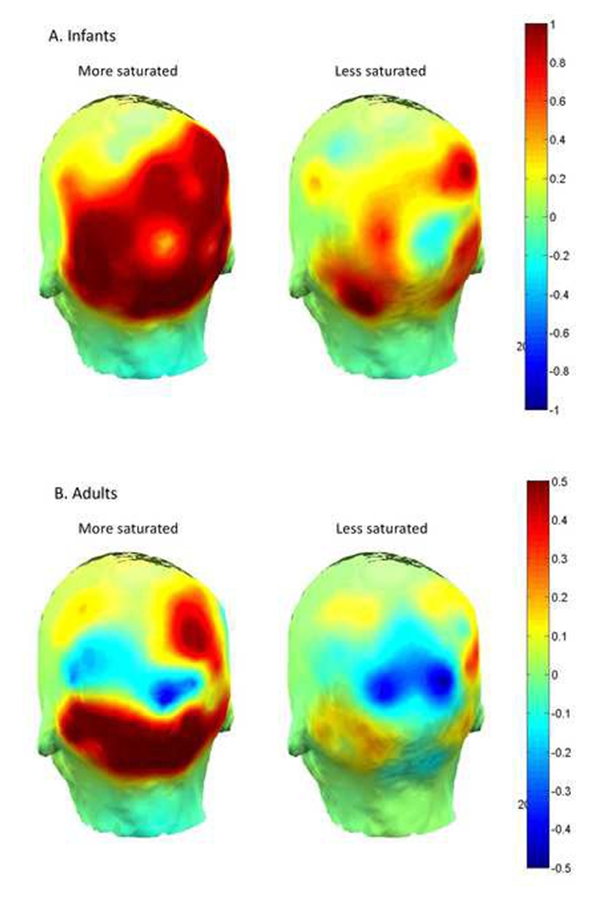
We studied visual perception in infants using EEG [Reference 1]. We presented infants with two levels of visual stimulus intensity, measured electrical activity at the infant cortex, and assessed infants’ preferential looking behavior. Chromatic saturation provided a convenient stimulus dimension to test the cascade because greater saturation is known to excite increased activity in the primate visual system and is generally thought to stimulate visual preference. Experiment 1 revealed that infants prefer (look longer) at the more saturated of two colors otherwise matched in hue and brightness. Experiment 2 showed increased aggregate neural cortical excitation in infants (and adults) to the more saturated of the same pair of colors. Thus, experiments 1 and 2 taken together confirm a cascade: visual stimulation of relatively greater intensity evokes relatively greater levels of bioelectrical cortical activity, which in turn is associated with relatively greater visual attention. As this cascade obtains near the beginning of life, it helps to account for early visual preferences and visual information processing.
Additionally, in collaboration with Anjana Bhat, we evaluated the effectiveness of various deep-learning models for classifying children with autism spectrum disorder (ASD) versus typically developing (TD) children, based on movement patterns observed during goal-directed arm tasks. IMU data (data from an inertial measurement unit [IMU], an electronic device that measures and reports a body's specific force, angular rate, and sometimes the orientation of the body) were collected from 41 children (age 6–17) performing a structured reaching task. Movement signals were preprocessed using normalization techniques and balanced datasets to ensure robust model evaluation. Models underwent K-fold cross-validation and patient-wise testing to measure their generalization capabilities. A variety of deep-learning models (Convolutional Neural Networks [CNNs], Long Short-Term Memory [LSTM] layers, and hybrid architectures [e.g., CNN-Autoencoder with LSTM]) were used to process IMU–derived time-series data. Among the models tested, the CNN-Autoencoder combined with LSTM layers delivered the best performance. Key findings include high accuracy (91.87%) for distinguishing ASD from TD children using movement patterns. LSTM layers improved performance significantly by leveraging sequence data effectively, and distinct movement features in ASD children, reinforcing motor assessment as a diagnostic tool.
COVID-19 point-of-care biosensor
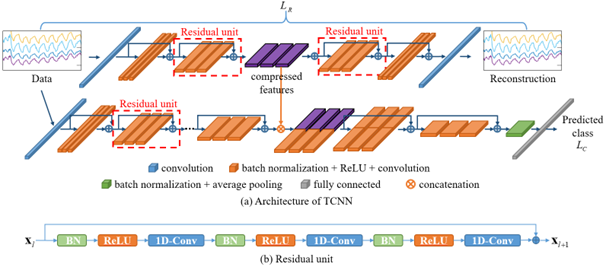
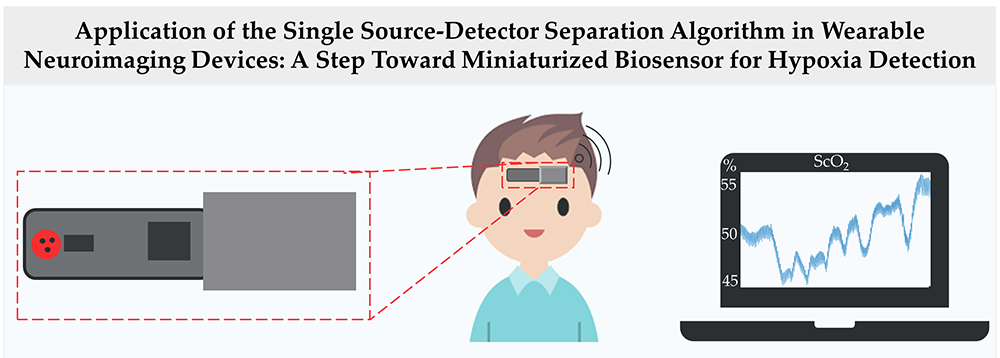
At the beginning of the global coronavirus pandemic in 2019 (COVID-19 disease), the COVID-19 multimodal biosensor project was established, which aimed to: (1) develop a wearable biosensor to monitor patients with infectious respiratory disease; and (2) classify breathing patterns in real-time using the measured data and a deep-learning algorithm. We developed a wireless multimodal biosensor, which can measure body temperature, heart rate, breathing rate, blood oxygen saturation, and tissue oxygen saturation. Using this biosensor, we collected data from 26 healthy volunteers during different breathing exercise under NICHD protocol #21CH0028. Data collected from the biosensor were inputted into a lab-developed two-stream convolutional neural network (TCNN) model to classify different breathing patterns. The TCNN consists of a convolutional neural network (CNN)–based autoencoder and classifier (Figure 2). The encoder of the autoencoder generates deep compressed feature maps, which contain the most important information constituting data. These maps are concatenated with feature maps generated by the classifier to classify breathing patterns. Using TCNN, we were able to obtain a classification accuracy 94.63% [Reference 5]. In addition, the proposed TCNN performed 2.6% better in terms of classification accuracy than a single-stream CNN (SCNN) [Park et al. Sensors 2023;23:5592]. Moreover, the TCNN mitigates the issue of declining learning performance with increasing network depth, as observed in the SCNN model. Our results demonstrate the robustness of the TCNN in classifying breathing patterns despite using a significantly smaller number of parameters and computations than do state-of-the-art classification models. In another application, we developed a single source-detector separation (SSDS) algorithm to detect hypoxia [Reference 4]. Hypoxia is characterized by reduced oxygen availability in tissue resulting from low blood supply or low blood oxygen saturation. The current standard method to monitor patients in an intensive care unit for hypoxia is arterial blood gas analysis (ABG), which requires blood samples to be drawn from a radial artery. The aim of the study is to use the SSDS to develop a miniaturized wearable biosensor to monitor cerebral tissue oxygenation, as well as to detect cerebral hypoxia (Figure 3). In this study, a breath-holding task was used to simulate change in cerebral tissue oxygenation (StO2). Data were collected from 26 healthy adult volunteers during a breath-holding task using a wearable NIRS device, which included two source–detector separations (SDSs). In the group analysis, both oxygenated-hemoglobin change and StO2 exhibited significant change during a breath-holding task. Specifically, they initially decreased to minimums at around 10 s and then steadily increased to maximums, which were significantly greater than baseline levels, at 25–30 s (p-HbO < 0.001 and p-StO2 < 0.05). However, at an individual level, the spatially resolved spectroscopy (SRS) method failed to detect changes in cerebral StO2 in response to a short breath-holding task. Furthermore, the SSDS algorithm is more robust than the SRS method in quantifying change in cerebral StO2 in response to a breath-holding task. In conclusion, our findings demonstrated the potential use of the SSDS algorithm in developing a miniaturized wearable biosensor to monitor cerebral StO2 and detect cerebral hypoxia.
Figure 2. Architecture of TCNN for breathing pattern classification
A. The autoencoder network is used to generate compressed features from the input data; then, the compressed features are combined with deep features extracted from the classification network.
B. The structure of a residual unit
Figure 2. Architecture of TCNN for breathing pattern classification
A. The autoencoder network is used to generate compressed features from the input data; then, the compressed features are combined with deep features extracted from the classification network.
B. The structure of a residual unit
Placenta oxygenation: from basics to point of care
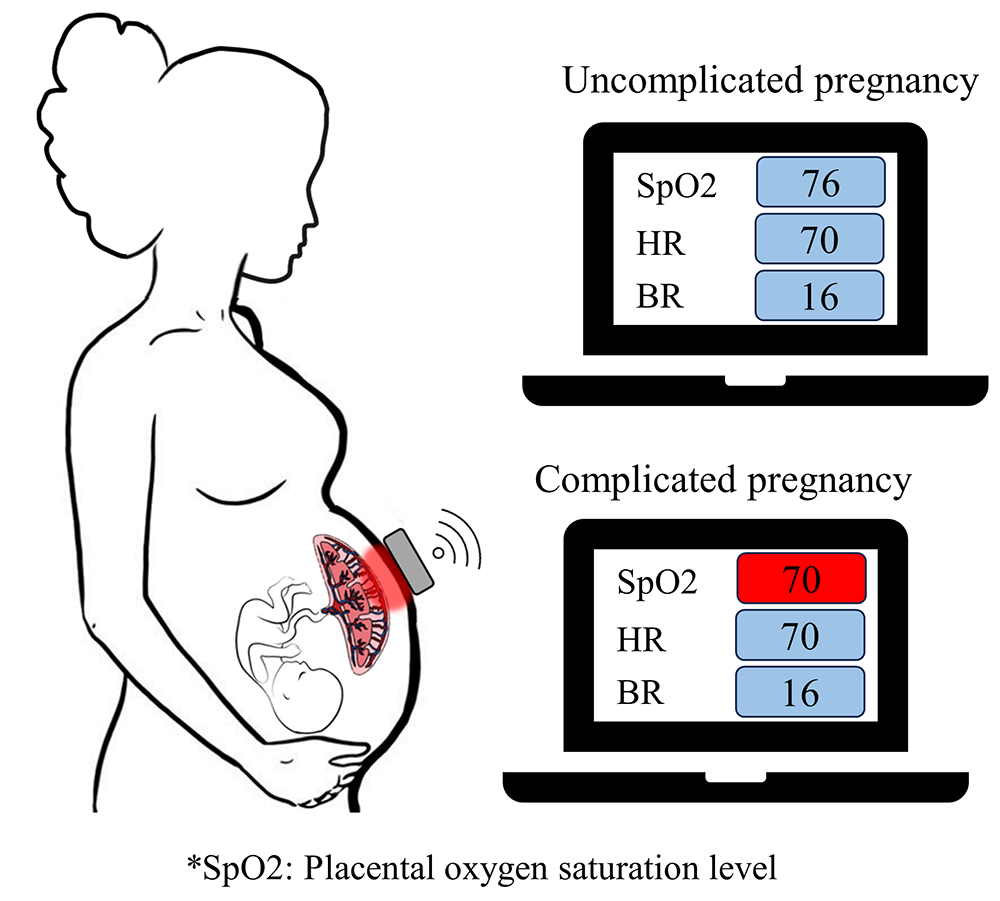
Intrauterine hypoxia is a critical condition in pregnancy and is characterized by a deficiency in oxygen supply to the fetus, which poses significant risks if undetected. Although hypoxia does not exert a predominant influence in early stages of embryonic development, existence in the second and third trimesters may cause abnormal fetal organogenesis and growth. If left untreated, it can result in redistribution of blood towards the fetal brain, heart, and upper extremities, which can eventually lead to a decline in cardiac function. In addition, it may cause an increased risk of hypertension, cardiovascular disease, abnormal central nervous system development, and cerebral palsy in the fetus. We developed two versions of a biosensor: Biosensor Version 1 (V.1) is a wearable device consisting of multiple near-infrared spectroscopy (NIRS) sensors; Biosensor Version 2 (V.2) is a wireless and wearable multimodal biosensor integrating a motion sensor and multiple NIRS sensors [Reference 4] (Figure 4). We conducted a pilot clinical study to assess the feasibility of the two biosensor versions in measuring transabdominal placental oxygenation and maternal physiological signals. Measurements of tissue oxygen saturation were compared with a commercial system (TRS-41 system, Hamamatsu Photonics, Japan) to validate performance. The placental oxygenation monitor yields oxygen saturation levels close to those from the commercial device (R2 = 0.94) with an averaged error of 2.7%. Biosensor V.1 was evaluated in 12 pregnant women in the third trimester and Biosensor V.2 was examined in 24 pregnant subjects in the second and third trimesters at the Perinatology Research Branch under protocol #090717MP4E, which was approved by the Wayne State University Human Investigations Committee Institutional Review Board (IRB). Among the study subjects, four participants had an uncomplicated pregnancy and 32 patients had either maternal preexisting conditions/complications, neonatal complications, and/or placental pathologic abnormalities. Patients with maternal conditions (69.5 ± 5.4 %), placental pathologic conditions (69.4 ± 4.9 %), and neonatal complications (68.0 ± 5.1 %) had statistically significantly lower transabdominal placental oxygenation levels than those without these conditions (76.0 ± 4.4 %) (F(3,104) = 6.6, p = 0.0004). Additionally, Biosensor V.2 could reliably detect maternal heart rate, respiratory rate, fetal movements, and uterine contractions. These findings demonstrated the feasibility of our novel multimodal biosensor in real-time continuous monitoring of transabdominal placental oxygenation and maternal physiological signals to detect at risk pregnancies and guide timely clinical intervention, thereby improving pregnancy outcomes.
Pediatric sleep study
We developed a wearable device designed to detect sleep apnea in children, specifically targeting the challenges of accurately monitoring oxygen saturation during sleep. This wireless Near-Infrared Spectroscopy (NIRS) device measures tissue oxygen levels, heart rate, and breathing patterns noninvasively, offering a more reliable alternative to traditional pulse oximeters, which can be inaccurate in certain populations. Currently, we are collaborating with Ashura Buckley and Bruce Tromberg to test this device on pediatric patients. The goal is to measure chest-tissue oxygen saturation during sleep apnea events and validate the results against clinical data. The device could improve the diagnosis and monitoring of obstructive sleep apnea (OSA) in children, offering a less invasive, more precise method for tracking the physiological impacts of OSA and guiding better treatment decisions. In addition, we conducted a systematic review to explore the effects of sleep apnea on children's psychological, developmental, and cognitive outcomes. Sleep apnea, often caused by obstructive airway issues such as enlarged tonsils, disrupts sleep patterns and oxygen levels, impairing brain development. Children with untreated sleep apnea are at higher risk of learning difficulties, behavioral issues, and poor academic performance. Our review aimed to fill gaps in the existing literature by examining these combined effects, and we will conduct a meta-analysis to synthesize the findings. Following this, we plan original research involving children with sleep apnea to assess these outcomes and inform clinical practices.
Total Body Photography for skin cancer research
Total Body Photography (TBP) is gaining popularity as a powerful tool for early melanoma detection. TBP allows monitoring of temporal changes in skin lesions, efficient screening of numerous lesions, and provides essential anatomical locations for dermatoscopic images. Despite its potential, several challenges are hindering its widespread application in skin cancer research. First, various digital imaging systems for TBP have been proposed, leading to an unmet need for a standardized data format for storing TBP data. To address this, we propose a ‘Work Item’ to create a data format compatible with the Digital Imaging and Communications in Medicine (DICOM) standard, allowing seamless integration with dermoscopy and future TBP systems. We verified the feasibility of the proposed TBP DICOM in an imaging-rich full-body scanning system. Secondly, finding lesion correspondence across full-body scans is essential for longitudinal tracking of skin lesions but remains underexplored. Therefore, we propose a novel framework combining geometric and texture information to localize skin-lesion correspondence from a source scan to a target scan in TBP. We evaluated the framework quantitatively on both a public and a private dataset, for which our success rates (at 10 mm criterion) are comparable to the only reported longitudinal study (Huang et al. ArXiv 2023:2307.09642v2). Third, the image quality (in terms of resolution and sharpness) for the total-body photography system does not have sufficient capabilities to automatically detect and analyze suspicious skin lesions. Therefore, we formulated the image-capture problem as limited to the depth of field of a camera, and we designed a novel algorithm to improve overall image quality. The proposed EM (expectation-maximization) and k-view algorithms improve the relative cost of the baseline single-view methods by at least 24% and 28% respectively, corresponding to increasing the in-focus surface area in total body photography by roughly 1550 cm2 and 1780 cm2 (Huang et al. 2024, submitted to WACV, 2025 [Winter Conference on Applications of Computer Vision, 2025]). Also, in collaboration with Mehran Armand and Jun Kang, we developed a novel shape-aware TBP system for high-resolution, quality-surface-coverage-maximized, and multi-spectral images. A clinical protocol was approved by the Johns Hopkins University IRB and allows the study to evaluate the clinical effectiveness of the proposed system, with 12 patients collected.
Additional Funding
- Bench to Bedside Award 345 (2016): “Mirror neuron network dysfunction as an early biomarker of neurodevelopment” (ongoing)
- Human Placenta Project–NICHD (2016, ongoing)
- Scientific Director's Award
Publications
- Visual stimulus structure, visual system neural activity, and visual behavior in young human infants. PLoS One 2024 19(6):e0302852
- Application of the single source–detector separation algorithm in wearable neuroimaging devices: a step toward miniaturized biosensor for hypoxia detection. Bioengineering (Basel) 2024 11(4):385
- A non-contacted height measurement method in two-dimensional space. Sensors (Basel) 2024 24(21):6796
- A wireless and wearable multimodal sensor to non-invasively monitor transabdominal placental oxygen saturation and maternal physiological signals. Biosensors (Basel) 2024 14(10):481
- Two-stream convolutional neural networks for breathing pattern classification: real-time monitoring of respiratory disease patients. Bioengineering (Basel) 2024 11(7):709
Collaborators
- Franck Amyot, PhD, Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD
- Mehran Armand, PhD, The Johns Hopkins University, Baltimore, MD
- Anjana Bhat, PhD, University of Delaware, Newark, DE
- Claude Boccara, PhD, École Supérieure de Physique et de Chimie Industrielles, Paris, France
- Ashura Buckley, MD, Sleep Disorders and Neurodevelopment Service, NIMH, Bethesda, MD
- Andrea Gropman, MD, Children's National Health System, Washington, DC
- Jun Kang, MD, The Johns Hopkins University, Baltimore, MD
- Jay Knutson, PhD, Laboratory of Molecular Biophysics, NHLBI, Bethesda, MD
- Guoyan Luo, MD, PhD, Howard University Hospital, Washington, DC
- Tom Pohida, MS, Division of Computational Bioscience, CIT, NIH, Bethesda, MD
- Randall Pursley, MSc, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Jessica C. Ramella-Roman, PhD, Florida International University, Miami, FL
- Roberto Romero-Galue, MD, D(Med)Sci, Perinatology Research Branch, NICHD, Detroit, MI
- Dan Sackett, PhD, Division of Basic and Translational Biophysics, NICHD, Bethesda, MD
- Babak Shadgan, MD, MSc, PhD, University of British Columbia, Vancouver, Canada
- Audrey Thurm, PhD, Pediatrics & Developmental Neuropsychiatry Branch, NIMH, Bethesda, MD
- Bruce Tromberg, PhD, Section on Biomedical Optics, NICHD, Bethesda, MD
- Eric Wassermann, MD, Cognitive Neuroscience Section, NINDS, Bethesda, MD
Contact
For more information, email gandjbaa@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/gandjbakhche.