Predictors of Bone Health in Adolescents and Young Adults
- Catherine Gordon,
MD, Head, Adolescent Bone & Body Composition Laboratory - Milena Jovanovic, PhD, Staff Scientist
- Gladys Andoh, FNP, CRNP, MS, Nurse Practitioner
- Devora Stein, MSN, CRNP, FNP, Nurse Practitioner
- Kitty Huntley, RT, Radiation Technician
- Jay Cheeti, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Alexandra Laufer, BA, Postbaccalaureate Intramural Research Training Award Fellow

The major aim of our lab is to understand factors that, during adolescence, impact bone density and skeletal strength during the adult years. We are examining how modifiable factors such as nutrition and physical activity influence the development of peak bone mass, as well as variables such as skin pigmentation and an individual’s genotype, which are determined at birth. In both healthy youth and those with chronic disease, we are exploring the interrelationship between body composition, circulating hormones, and bone marrow adiposity and its effect on bone turnover and skeletal accrual.
A focus of our research is how physical and emotional health are compromised in adolescents and young women with premature ovarian insufficiency (POI). POI presents along a broad clinical spectrum. We are interested in both the presentation and causes of POI, including those seen in childhood cancer survivors, and ovarian dysfunction resulting from autoimmune, metabolic, genetic/syndromic, and idiopathic (unknown) causes. We are conducting a natural history study to characterize numerous health outcomes and are launching a clinical trial to identify the optimal estrogen replacement regimen for adolescents and young women with this diagnosis. We are also employing novel tools to provide state-of-the-art assessments of bone density, body composition, and skeletal strength.
In addition, we are interested in understanding the role of androgens in bone development by investigating the skeletal phenotype of patients with the androgen insensitivity syndrome (AIS) by obtaining assessments in a natural history study of these patients, in collaboration with Veronica Gomez-Lobo.
Our lab is also interested in the skeletal phenotype associated with rare genetic diagnoses, some of which resemble or meet criteria for a skeletal dysplasia. Examples include progeria (Hutchinson-Gilford progeria syndrome), Ollier disease, and Maffucci syndrome. Ollier disease and Maffucci syndrome are disorders associated with skeletal abnormalities and enchondromas, which are benign tumors of cartilage that usually develop in the metaphyseal regions of bone. Currently, the only treatment for these patients involves surgical removal of oversized enchondromas. In collaboration with Nara Sobreira, we are conducting a natural history study to examine the skeletal phenotype and genetic background of children with Ollier disease and Maffucci syndrome so as to understand the molecular mechanisms underlying their disease.
We are also conducting studies to understand the relationship between bone mineral density (BMD), skin pigmentation, and underlying genetic factors with the aim of moving away from race/ethnicity in the interpretation of BMD.
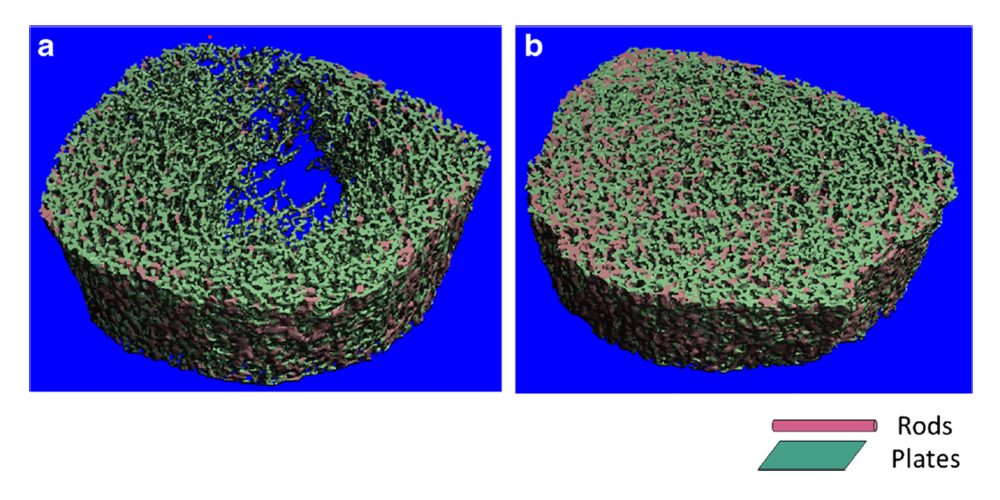
High-resolution peripheral computed tomography
High-resolution peripheral computed tomography (HRpQCT) is a bone assessment modality that affords measurements of the appendicular (peripheral) skeleton, as well as evaluation of bone microarchitecture and skeletal strength. As a non-invasive tool, it is ideal for obtaining measurements in the pediatric and adolescent population. Our lab is one of a relatively small number of centers that has an HRpQCT scanner. In a number of chronic disease models, we are examining the relation between failure load and other HRpQCT–derived outcomes and both bone density and fracture risk.
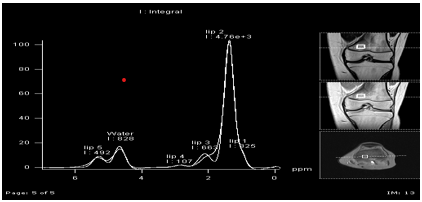
Evaluations of bone marrow composition
Our team is using magnetic resonance (MR) imaging and spectroscopy to evaluate bone marrow fat, which is directly influenced by hormonal signals. We have studied bone marrow composition in adolescents with anorexia nervosa, and we are employing the technique to examine the correlation between marrow fat and bone accrual in adolescents with inflammatory bowel disease and other pediatric clinical models. T1 maps (pixel-by-pixel method of quantifying T1 relaxation time) and MR spectroscopy evaluations afford non-invasive means to evaluate bone marrow composition in children and adolescents.
Additional Funding
- U01HD107957-01 (Sobreira/Gordon): Delineation of the natural history of Ollier disease and Maffucci syndrome and investigation of their genetic bases
Publications
- Characterizing the adolescent premature ovarian insufficiency phenotype: a case control study. J Pediatr Adolesc Gynecol 2022 36:122–127
- Evaluation and management of primary ovarian insufficiency in adolescents and young adults. J Pediatr Adolesc Gynecol 2018 31:13–18
- Extraskeletal calcifications in Hutchinson-Gilford progeria syndrome. Bone 2019 125:103–111
- Bone marrow adiposity in pediatric Crohn's disease. Bone 2022 162:116453
- Evaluation and management of amenorrhea. JAMA 2021 326:1962–1963
Collaborators
- Leslie Bardin, MS, OTR/L, Occupational Therapy, NIH Clinical Center, Bethesda, MD
- Allessandra Brofferio, MD, Cardiovascular Branch, NHLBI, Bethesda, MD
- Tom Burklow, MD, Office of Clinical Research Training and Medical Education, NIH Clinical Center, Bethesda, MD
- Amy DiVasta, MD, MMSc, Boston Children's Hospital, Boston, MA
- Veronica Gomez-Lobo, MD, Pediatric and Adolescent Gynecology, NICHD, Bethesda, MD
- Rebecca J. Gordon, MD, Boston Children's Hospital, Boston, MA
- Hanna Hildenbrand, MS, OTR/L, Occupational Therapy, NIH Clinical Center, Bethesda, MD
- Bonnie Hodsdon, BS, OTR/L, Occupational Therapy, NIH Clinical Center, Bethesda, MD
- Heidi Kalkwarf, PhD, RD, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
- Jacqueline Maher, MD, FACOG, Pediatric and Adolescent Gynecology, NICHD, Bethesda, MD
- Robert Shamburek, MD, Cardio-Pulmonary Branch, NHLBI, Bethesda, MD
- Nara Sobreira, MD, PhD, Johns Hopkins School of Medicine, Baltimore, MD
Contact
For more information, email catherine.gordon@nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/gordon.