Innovative Approaches to Clinical Management of Complex Pregnancy Issues
- Katherine L. Grantz,
MD, MS, Senior Investigator, Epidemiology Branch, DiPHR - Jessica Gleason, PhD, Staff Scientist
- Kathryn Wagner, PhD, Postdoctoral Intramural Research Training Award Fellow
- Alexandra Jean-Louis, BS, Postbaccalaureate Intramural Research Training Award Fellow, Postbaccalaureate Enrichment Program (OITE-PEP)

Novel measures of fetal growth and development: state-of-the-art tools to assess fetal health
Normal fetal growth is a marker of an optimal intrauterine environment and is important for long-term offspring health. Fetal size is monitored during pregnancy because fetal growth restriction and small-for-gestational-age as well as macrosomia and large-for-gestational-age are associated with increased risk of perinatal morbidity and mortality. Yet distinguishing fetal growth that is constitutionally small or large from growth that is pathologically restricted or increased presents a significant challenge in obstetrics. Cross-sectional fetal measurements are typically compared with reference size-for-age curves, with a range of 10th–90th percentiles considered appropriate for gestational age. Yet, a single measurement can only indicate size. At least two measurements separated in time are needed to estimate actual fetal growth. Fetal growth velocity is the rate of fetal growth over a given time interval (e.g., g per week). Understanding whether fetal growth has deviated from a normal trajectory may have more clinical utility to distinguish constitutional from pathologic fetal growth than using a particular threshold of fetal size from a single time measure. Fetal growth, along with accurate gestational dating, are key factors in clinical decision making for antenatal monitoring and determining the route and timing of delivery. Yet until recently, there has been a lack of longitudinal prospective studies with diverse populations that have collected repeated ultrasound measurements. In addition, error in ultrasound estimated fetal weight (EFW) prediction of birth weight has clinical implications such as timing of delivery for fetal undergrowth or decision for cesarean due to suspected macrosomia. Our research program is addressing these critical data gaps using several approaches, including developing and applying fetal growth standards for use in clinical practice, investigating the role of fetal growth velocity, customized and individualized fetal growth references, and fetal 3D volumes.
The NICHD Fetal Growth Studies team was responsible for a multidisciplinary effort that generated fetal growth percentile charts in a diverse U.S. population for clinical practice [Buck Lewis et al. Am J Obstet Gynecol 2015;213:449; Grantz et al. Am J Obstet Gynecol 2022;226:576]. Our team developed the first ever fetal growth velocity calculator for clinical use [Grantz et al. Am J Obstet Gynecol 2018;219:28; Grantz et al. Am J Obstet Gynecol 2022;227:916]. The benefits of using growth velocity to categorize fetal growth and assess its contribution to birth weight had not been empirically demonstrated prior to our work in this area.
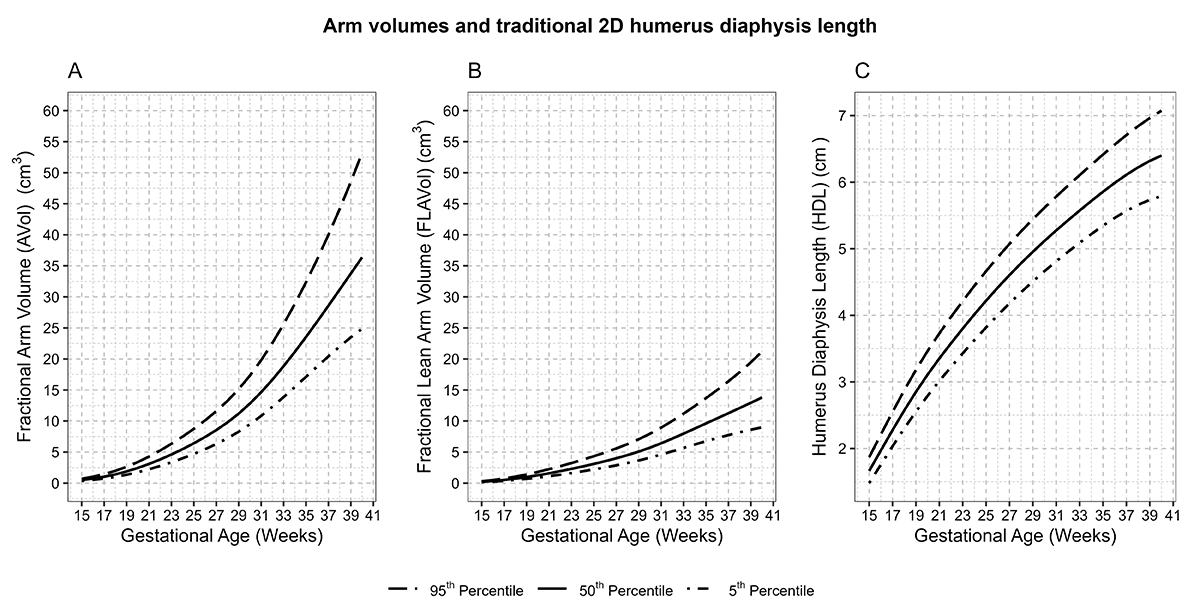
In the Fetal 3D Study, our group is among the first to have accumulated the largest collection of fetal 3-dimensional (3D) volumes from a racially and ethnically diverse pregnancy cohort with repeat ultrasounds spanning the length of gestation. While 3D ultrasound capability is generally available, it has not been widely adopted into clinical practice. This past year, our team found that intra- and inter-rater agreement had high reliability for many structures, which is an important initial step for integrating into clinical care [Reference 2]. We also established standards for fetal soft-tissue and organ-volume measurements by 3D ultrasound technology [Reference 1]. Growth patterns, timing of maximal growth for three-dimensional lean and fat measures, and limb and organ volumes differed from patterns revealed by traditional 2D growth measures. For example, fractional arm and lean arm volumes, depicted in panels A and B of Figure 1, increased with acceleration around 29–30 weeks. In contrast, the 2D humerus diaphysis length demonstrated a logarithmic growth curve, with fastest growth from 16–26 weeks. These findings suggest that novel 3D fetal growth parameters reflect unique facets of fetal growth. Ongoing work is investigating whether growth in these 3D measures may be altered by genetic, nutritional, metabolic, or environmental influences and pregnancy complications, in ways not identifiable using corresponding 2D measures.
Figure 1. Arm volumes and traditional 2D humerus diaphysis length
Distribution of A: Fractional arm volume (AVol) (cm3); B: Fractional lean arm volume (FLAVol) (cm3); C: Humerus length (cm) and gestational age, NICHD Fetal 3D Study. Estimated 5th, 50th, and 90th percentiles for measurements were estimated from linear mixed models with log-transformed outcomes and cubic splines and weighted for self-reported race and ethnicity using 2011 natality data [Reference 1].
Figure 1. Arm volumes and traditional 2D humerus diaphysis length
Distribution of A: Fractional arm volume (AVol) (cm3); B: Fractional lean arm volume (FLAVol) (cm3); C: Humerus length (cm) and gestational age, NICHD Fetal 3D Study. Estimated 5th, 50th, and 90th percentiles for measurements were estimated from linear mixed models with log-transformed outcomes and cubic splines and weighted for self-reported race and ethnicity using 2011 natality data [Reference 1].
Another area lacking data to guide clinical practice and targeted by our research program is twin pregnancies. Defining abnormal fetal growth in twins is more complicated than for singletons, given that growth restriction can affect one or both twins and can also be defined in terms of discordance. In the NICHD Fetal Growth Studies – Dichorionic Twins, our team developed twin fetal growth percentile charts [Grantz et al. Am J Obstet Gynecol 2016;215:221]. We found that fetal growth of twins started to deviate from that of singletons in the 3rd trimester. While it has been established that twins are smaller on average than that of singletons at birth, it was unknown whether growth patterns differed in infancy and childhood. Our team followed up on this work and found that, while twins grew more rapidly in infancy than singletons, they did not have an increased risk of obesity in mid-childhood [Reference 3]. These findings are reassuring for parents and providers in that “catch-up” growth is normal for twins. In early childhood (up to 3 years), twins remained smaller in height and weight than singletons. By mid-childhood (7–9 years), differences in height and weight between twins and singletons were lower. Work is ongoing to combine data from available studies to investigate growth in twins through adulthood.
Timing of delivery for pregnancies with complications
Much attention has focused on preterm delivery, but level A evidence to guide delivery timing in pregnancies with complications is lacking, an important data gap highlighted by a 2011 joint NICHD workshop. Dr. Grantz is co-PI of the Study of Pregnancy and Neonatal Health (SPAN), leading the TIMing of dElivery (TIME) trial to determine the optimal timing of delivery for uncontrolled gestational diabetes mellitus–complicated pregnancies. Recruitment is ongoing.
Labor and delivery management
Our team is also addressing labor and delivery management to prevent medically unnecessary cesarean deliveries, an issue declared as a national priority, given that cesarean delivery is a risk factor for severe maternal morbidity and mortality. The work uses data from the Consortium on Safe Labor Study. The American College of Obstetricians and Gynecologists released new guidelines this past year on management of labor, and included recommendations specific to the second stage of labor that were heavily informed by a previous publication by our team on maternal and neonatal outcomes associated with a prolonged second stage of labor [Laughon et al. Obstet Gynecol 2014;124:57]. The reason that we are targeting efforts to prolonged second stage is because, in other prior work, we found that cesareans performed in the second stage of labor are a major contributor to excess cesarean rates [Boyle et al. Obstet Gynecol 2013;122:33]. Ongoing collaborative work with the Biostatistics and Bioinformatics Branch uses modern statistical approaches not previously used in labor research to build predictive algorithms to apply to the areas of second-stage labor management.
Publications
- Multiethnic growth standards for fetal body composition and organ volumes derived from 3D ultrasonography. Am J Obstet Gynecol 2024 Epub ahead of print
- The NICHD Fetal 3D Study: a pregnancy cohort study of fetal body composition and volumes. Am J Epidemiol 2024 193:580–595
- Longitudinal child growth patterns in twins and singletons in the Upstate KIDS cohort. J Pediatr 2023 263:113720
- Comparing population-based fetal growth standards in a US cohort. Am J Obstet Gynecol 2024 231(3):338.e1–338.e18
- History of multifetal gestation and long-term maternal mortality. Paediatr Perinat Epidemiol 2024 38:219–226
Collaborators
- Joseph Biggio, MD, Ochsner Health, Jefferson, LA
- Zhen Chen, PhD, Biostatistics and Bioinformatics Branch, Division of Population Health Research, NICHD
- Celeste Durnwald, MD, Penn Medical, Philadelphia, PA
- Una Grewal, PhD, Division of Population Health Research, NICHD, Bethesda, MD
- Brenna Hughes, MD, Duke University School of Medicine, Durham, NC
- George L. Maxwell, MD, Inova Health System, Fairfax, VA
- Daniel Molina, PharmD, MBA, Technical Resources International, Inc., Bethesda, MD
- Jessica Page, MD, Intermountain Health, Salt Lake City, UT
- Diane Putnick, PhD, Epidemiology Branch, Division of Population Health Research, NICHD, Bethesda, MD
- Robert Silver, MD, University of Utah, Salt Lake City, UT
- Maddy St. Ville, PhD, Biostatistics and Bioinformatics Branch, Division of Population Health Research, NICHD, Bethesda, MD
- Rajeshwari Sundaram, PhD, Biostatistics and Bioinformatics Branch, Division of Population Health Research, NICHD, Bethesda, MD
- Fasil Tekola-Ayele, PhD, Epidemiology Branch, Division of Population Health Research, NICHD, Bethesda, MD
- John Thorp, MD, University of North Carolina, Chapel Hill, NC
- Alan Tita, MD, The University of Alabama at Birmingham, Birmingham, AL
- Edwina Yeung, PhD, Epidemiology Branch, Division of Population Health Research, NICHD, Bethesda, MD
Contact
For more information, email katherine.grantz@nih.gov or visit https://irp.nih.gov/pi/katherine-grantz.