Molecular Nature and Functional Role of Dendritic Voltage-Gated Ion Channels
- Dax Hoffman,
PhD, Head, Section on Molecular Neurophysiology and Biophysics - Jiahua Hu, PhD, Staff Scientist
- Lin Lin, PhD, Microbiologist
- Ying Liu, MD, Biologist
- Cole Malloy, PhD, Postdoctoral Fellow
- Meghyn Welch, PhD, Postdoctoral Fellow
- Ashley Pratt, BS, PhD Student
- Kailey Jerome, BS, Postbaccalaureate Fellow

The central nervous system (CNS) underlies all our experiences, actions, emotions, knowledge, and memories. With billions of neurons each firing hundreds of times per second, the complexity of the brain is stunning. To pare down the task of understanding something so complex, our research approach calls for studying the workings of a single central neuron: the pyramidal neuron from the CA1 region of the hippocampus. In humans, the hippocampus is essential for long-term memory and is among the first brain regions affected by epilepsy and Alzheimer’s disease. To understand how the hippocampus stores and processes information, we focus on the CA1 pyramidal neuron, one of its principal cell types. Each of these cells receives tens of thousands of inputs onto its dendrites, and it is commonly thought that information is stored by altering the strength of individual synapses (synaptic plasticity). Recent evidence suggests that the regulation of synaptic-surface expression of glutamate receptors can, in part, determine synaptic strength. However, the dendrites contain an abundance of ion channels that are involved in receiving, transforming, and relaying information in the dendrites, adding an additional layer of complexity to neuronal information processing.
We found that the A-type potassium channel subunit Kv4.2 is highly expressed in the dendritic regions of CA1 neurons in the hippocampus and, as one of the primary regulators of dendritic excitability, plays a pivotal role in information processing. Kv4.2 is targeted for modulation during the types of plasticity thought to underlie learning and memory. Moreover, we found that the functional expression level of Kv4.2 regulates the subtype expression of NMDA–type glutamate receptors, the predominant molecular devices controlling synaptic plasticity and memory. We are currently following up on these findings with more detailed investigations into the mechanisms of activity-dependent Kv4.2 regulation. In addition, we have begun to investigate the role of dendritic voltage-gated potassium and calcium channels in neuronal development and developmental disorders.
Role of voltage-gated ion channels in synaptic development and disease
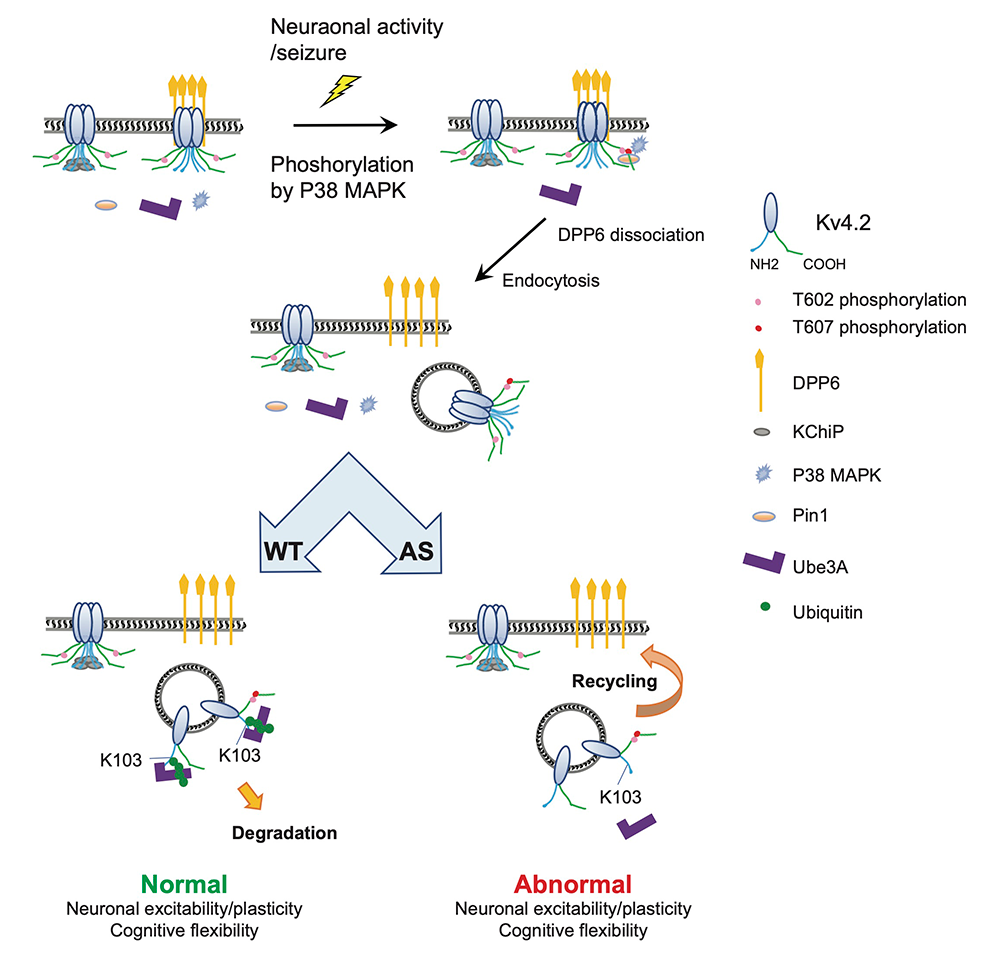
Kv4.2 is an activity-dependent Ube3A substrate and contributes to synaptic plasticity and cognitive flexibility in Angelman syndrome.
Angelman syndrome (AS) is a severe neurodevelopmental disorder, affecting 1 in 20,000 people, caused by loss of function of imprinted genes on chromosome 15q11–13 or mutations in Ube3A. Ube3A is expressed exclusively from the maternal allele in hippocampal neurons and cerebellar Purkinje cells. Loss of Ube3A function leads to accumulation of target proteins, disrupting neuronal function. A tandem affinity purification–mass spectrometry (TAP–MS) screen identified Ube3A as a Kv4.2–binding protein. Follow-up studies confirmed activity-dependent Kv4.2–Ube3A interaction, showing that Ube3A ubiquitinates Kv4.2 at residue K103, which is required for activity-induced Kv4.2 degradation.
In AS mouse models, Kv4.2 protein levels and K+ currents are elevated in the hippocampus. Seizure-induced Kv4.2 degradation, which normally requires Ube3A, is absent in AS mice. Ube3A–mediated Kv4.2 ubiquitination is significantly reduced in AS hippocampi, further supporting the role of Ube3A in Kv4.2 degradation. Additionally, studies showed that seizure-induced Kv4.2 degradation occurs on dipeptidyl peptidase-like 6 (DPP6)–containing Kv4.2 complexes (see below), requiring Kv4.2 phosphorylation at the T602/7 Pin1 site.
Patch-clamp studies revealed deficits in mini excitatory postsynaptic potential (mEPSC) frequency and spike-timing–dependent long-term potentiation (LTP) in AS mice, which were rescued by crossing AS mice with conditional Kv4.2 knockout (KO) (Kv4.2cKO) mice. Behavioral tests showed that some locomotion, nesting, and learning impairments in AS mice were also partially rescued in AS/Kv4.2cKO mice. The findings reveal a novel Ube3A downstream pathway regulating synaptic plasticity and cognitive behaviors, offering potential targets for AS treatment.
Kv4.2 complex regulation and its role in cognitive flexibility/isomerase regulation of K+ channel trafficking and function
Cole Malloy is investigating the mechanisms underlying enhanced cognitive flexibility in Kv4.2TA mice, the first mouse model with this phenotype. Focusing on synaptic differences between Kv4.2TA and wild-type (WT) mice, patch-clamp electrophysiology in hippocampal CA1 pyramidal cells showed that Kv4.2TA mice have similar basal synaptic transmission and preserved long-term plasticity. However, they exhibit a significant enhancement in the reversal of spike-timing–dependent long-term potentiation (STD–LTP) with low-frequency stimulation (LFS), suggesting a synapse state–dependent difference in synaptic plasticity attributable to altered Kv4.2 complex regulation.
Pharmacological manipulations during LFS revealed distinct mechanisms driving this metaplasticity. The NMDA antagonist 5-AP, which fully blocks depotentiation in WT mice, only partially blocks it (about 40%) in Kv4.2TA mice, indicating an additional mechanism in Kv4.2TA mice. The hypothesis is that altered metabotropic glutamate-receptor (mGluR) signaling, specifically mGluR5, underlies this enhancement. Co-application of the mGluR5 antagonist MTEP with 5-AP fully rescues the depotentiation in Kv4.2TA mice, bringing it to WT levels, while MTEP alone fully prevents depotentiation in Kv4.2TA mice and only partially affects WT mice, suggesting that depotentiation in Kv4.2TA mice is primarily driven by mGluR5, while in WT mice NMDA receptors are more dominant.
In summary, our study uncovers a novel metaplasticity mechanism in Kv4.2TA mice linked to cognitive flexibility, with implications for therapeutic strategies targeting neurodevelopmental disorders with cognitive flexibility impairments. It reveals a shift in the molecular drivers of depotentiation between Kv4.2TA and WT mice, likely attributable to impaired Kv4.2 complex trafficking during synaptic activity.
Preso1 regulation of Kv4.2 channels in the hippocampus
Intellectual disability (ID) affects 1–3% of the population and is marked by learning and adaptive behavior impairments. While the cause is unclear, mutations in the Preso1 (FRMPD4) gene, a neuronal post-synaptic scaffold protein, have been linked to X-linked ID. Preso1 regulates Kv4.2 channels in the hippocampus. We demonstrated that Preso1 directly binds to Kv4.2. Meghyn Welch found that knocking out Preso1 reduced the transient A-type potassium current (IA) by about 30% in CA1 pyramidal neurons compared with controls. The reduction in Kv4.2 channels led to increased hippocampal excitability, with KO mice showing higher firing rates, a more depolarized action-potential threshold, higher action-potential amplitude, lower rheobase, and shorter latency to fire. The synaptic properties in KO mice showed no difference in paired-pulse ratio, but a significant deficit in spike-timing–dependent LTP at CA3–CA1 synapses compared with wild-type mice. Ongoing research is examining basal synaptic transmission. Our findings identify Kv4.2 as a novel target of Preso1, showing that its disruption affects hippocampal excitability and synaptic plasticity in Preso1 KO mice.
Seizure analysis in aging DPP6-KO mouse related to Alzheimer’s disease/dementia
We discovered novel roles for the voltage-gated potassium channel auxiliary subunit DPP6 (dipeptidyl peptidase-like 6) in neuronal development, learning, memory, and its connection to Alzheimer's disease (AD)/dementia. In aging DPP6–KO mice, amyloid-β (Aβ)–associated structures were found in the hippocampal area CA1, likely from degenerating presynaptic terminals, with a higher prevalence compared than in WT mice. The KO mice also showed increased Aβ, tau pathologies, neuroinflammation, and sleep disturbances.
AD patients have an eight-fold higher risk of epileptic seizures. We examined seizures in DPP6–KO mice, detecting EEG spike-wave discharges, indicative of nonconvulsive seizures. Using HD-X02 telemetry, which permits in vivo monitoring of neuronal activity, data from 12-month-old DPP6–KO mice showed a higher prevalence of spike-wave discharges and nonconvulsive seizures than in WT controls. They also had longer spike-train durations and more high-amplitude single spikes. However, 3-month-old DPP6–KO mice did not exhibit significant increases in seizures compared with WT littermates, findings that suggest age-dependent seizure activity and high-amplitude spikes in DPP6–KO mice, reinforcing DPP6's role in Alzheimer's/dementia.
Publications
- R-type voltage-gated Ca2+ channels mediate A-type K+ current regulation of synaptic input in hippocampal dendrites. Cell Rep 2022 38(3):110264
- Alzheimer's disease/dementia-associated brain pathology in aging DPP6-KO mice. Neurobiol Dis 2022 174:105887
- Neuronal roles of the multifunctional protein dipeptidyl peptidase-like 6 (DPP6). Int J Mol Sci 2022 23(16):9184
- Activity-dependent isomerization of Kv4.2 by Pin1 regulates cognitive flexibility. Nat Commun 2020 11(1):1567
- P38 regulates kainic acid-induced seizure and neuronal firing via Kv4.2 phosphorylation. Int J Mol Sci 2020 21(16):5921
- A novel structure associated with aging is augmented in the DPP6-KO mouse brain. Acta Neuropathol Commun 2020 8(1):197
Collaborators
- Heather Cameron, PhD, Section on Neuroplasticity, NIMH, Bethesda, MD
- Thomas B. Friedman, PhD, Section on Human Genetics, NIDCD, Bethesda, MD
Contact
For more information, email hoffmand@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/hoffman.