The Role of Metabolism in the Regulation of Oocyte Development and Growth
- Mary Lilly,
PhD, Head, Section on Gamete Development - Chun-Yuan Ting, PhD, Staff Scientist
- Kuikwon Kim, MS, Biologist
- Lucia Bettedi, PhD, Visiting Fellow
- Shu Yang, PhD, Postdoctoral Fellow
- Richard Garcia, BS, Postbaccalaureate Fellow
- Natalie Rowland, BS, Postbaccalaureate Fellow

Our long-term goal is to obtain a comprehensive understanding of how metabolic signaling pathways influence oocyte growth, development, and quality. Chromosome mis-segregation during female meiosis is the leading cause of miscarriages and birth defects in humans. Recent evidence suggests that many meiotic errors occur downstream of defects in oocyte growth and/or in the hormonal signaling pathways that drive differentiation of the oocyte. Thus, understanding how oocyte metabolism and growth impact meiotic progression is essential to studies in both reproductive biology and medicine.
Metabolic dysregulation, including obesity and diabetes, negatively impact human fertility. We use the genetically tractable model organism Drosophila melanogaster to examine how meiotic progression and oocyte development are instructed by the metabolic program of the egg. In mammals, studies on the early stages of oogenesis face serious technical challenges in that entry into the meiotic cycle, meiotic recombination, and the initiation of the highly conserved prophase I arrest all occur during embryogenesis. By contrast, in Drosophila these critical, highly conserved events of early oogenesis all take place continuously within the adult female. Easy access to the early stages of oogenesis, coupled with available genetic and molecular-genetic tools, makes Drosophila an excellent model for studies on the role of metabolism in oocyte development and maintenance.
Additionally, we also utilize the Drosophila ovary as a model to investigate the tissue-specific regulation of metabolism. Our focus is on gaining a molecular understanding of how the GATOR complex modulates TORC1 activity and regulates the autophagic-lysosomal pathway.
The GATOR complex: integrating developmental and metabolic signals in oogenesis
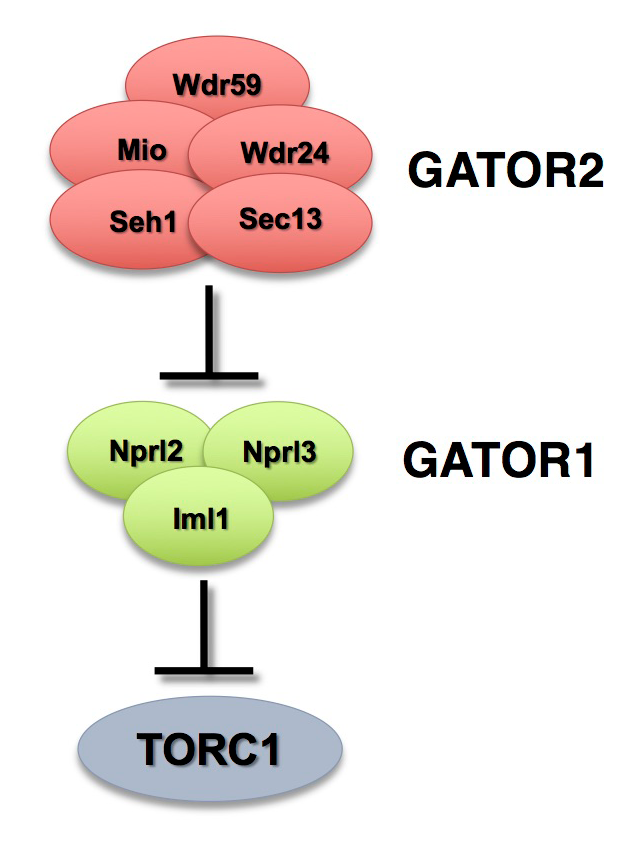
The Target of Rapamycin Complex 1 (TORC1) regulates cell growth and metabolism in response to many inputs, including amino acid availability and intracellular energy status. In the presence of sufficient nutrients and appropriate growth signals, the Ragulator and the Rag GTPases (a complex that regulates lysosomal signaling and trafficking) target TORC1 to lysosomal membranes, where TORC1 associates with its activator, the small GTPase Rheb. Once activated, TORC1 is competent to phosphorylate its downstream targets. The Gap activity towards Rags (GATOR) complex is an upstream regulator of TORC1 activity. The GATOR complex consists of two subcomplexes (Figure 1). The GATOR1 complex inhibits TORC1 activity in response to amino acid starvation and is a trimeric protein complex consisting of the proteins Nprl2, Nprl3, and Iml1. Evidence from yeast and mammals indicates that the components of the GATOR1 complex function as GTPase–activating proteins (GAP), which inhibit TORC1 activity by inactivating the Rag GTPases. Notably, Nprl2 and Iml1 are tumor-suppressor genes, and mutations in Iml1, known as DEPDC5 in mammals, are a leading cause of hereditary epilepsy.
Our work, as well as that of others, found that the GATOR2 complex activates TORC1 by opposing the TORC1–inhibitory activity of GATOR1. Intriguingly, computational analysis indicates that the GATOR2 components Mio and Seh1, as well as several other members of the GATOR2 complex, have structural features consistent with coatomer proteins and membrane-tethering complexes. In line with the structural similarity to proteins that influence membrane curvature, we showed that three components of the GATOR2 complex, Mio, Seh1, and Wdr24, localize to the outer surface of lysosomes, the site of TORC1 regulation. However, how GATOR2 inhibits GATOR1 activity, thus allowing for the robust activation of TORC1, remains unknown. Additionally, the role of the GATOR1 and GATOR2 complexes in both the development and physiology of multicellular animals remains poorly defined. Over the past year, we used molecular, genetic, and cell-biological approaches to define the in vivo functions of the GATOR1 and GATOR2 complexes in both Drosophila and mammals. Additionally, we examined how the regulation of metabolism during oogenesis by the GATOR1 complex impacts oocyte development and the maintenance of oocyte quality.
The GATOR2 complex maintains lysosomal-autophagic function by inhibiting the degradation of MiT/TFE transcription factors.
The lysosomal–autophagic system regulates the balance between anabolism and catabolism, which is essential to metabolic homeostasis. During periods of nutrient stress, cells upregulate lysosomal function and the autophagic process to promote the catabolic breakdown of macromolecules, such as proteins and lipids, to provide building blocks for cells to reuse. The MiT/TFE family promotes lysosomal-autophagic function by stimulating the transcription of numerous genes related to the lysosomal-autophagic system, including lysosomal lumen enzymes, V-ATPase, and ATG proteins. A large body of work has provided a detailed mechanism for how TORC1 functions to inhibit the activation of MiT/TFE proteins through multiple mechanisms, including cytoplasmic retention and proteolytic degradation. These regulatory pathways allow MiT/TFEs to transcribe the genes required to promote catabolic metabolism during periods of nutrient stress, when TORC1 activity is low.
We discovered that the GATOR2 complex has independent roles in TORC1 regulation and MiT/TFE protein protection and is thus central to coordinating cellular metabolism with the control of the lysosomal-autophagic system. We found that, in cells with deletions of the GATOR2 subunits WDR24, MIOS or SEH1, but not WDR59, members of the MiT/TFEs family of transcription factors TFEB, TFE3 and MITF are ubiquitylated and degraded by the proteasome. Low levels of MiT/TFEs result in a reduction in the transcription of a wide range of lysosomal-autophagic genes, including V-ATPase subunits, lysosomal enzymes (Cathepsins), structure proteins (LAMPs and LAMTORs) and several ATG genes (ATG9), causing a significantly lowered lysosomal pH and a systematic failure in digestion of lysosomal cargoes. We identified a trio of E3 ligases, HERC2, UBE3A and STUB1, that target MiT/TFEs for ubiquitin-mediated degradation in GATOR2 knockout (KO) cells. We also found that, in autophagy-addicted pancreatic ductal adenocarcinoma (PDAC) cells, the GATOR2 complex is essential for maintaining PDAC cell proliferation, invasion, and malignancy by fulfilling its dual roles in cell metabolism, i.e., activation of TORC1 and protection of MiT/TFEs.
To complement the above studies, we recently knocked down the GATOR2 component WDR24 in two kidney-cancer-cell models, UOK257 and UOK124. The UOK 257 cell line was derived from a human renal carcinoma of an individual with Birt-Hogg-Dubé (BHD) syndrome, which results from a mutation in the FLCN gene. FLCN regulates lysosome function by promoting the TORC1–dependent phosphorylation and cytoplasmic sequestration of TFEB. In the absence of FLCN, TFEB is not recruited to the lysosome but instead enters the nucleus and is active. The UOK124 cell line was derived from a Xp11.2 translocation renal cell carcinoma (tRCC). Xp11.2 tRCCs represent an aggressive type of kidney cancer resulting from genomic rearrangements of TFE3 that produce oncogenic TFE3 fusion proteins. Our preliminary data indicate that WDR24 knockdown results in markedly decreased levels of TFEB and TFE3 in UOK257 and UOK124 tRCC cells. We will continue to analyze how GATOR2 impacts these two cancer cell lines using strategies similar to those we employed to follow MiT/TFE levels and function in HeLa and PDAC cells. Our data indicate that the GATOR2 complex acts upstream of the TORC1 regulation of MiT/TFE activity. Thus, the GATOR2 complex may be an attractive target for chemotherapeutic intervention.
The GATOR2 component Wdr59 promotes or inhibits TORC1 activity depending on cellular context.
We defined two novel functions for the GATOR2 component Wdr59. Studies performed in tissue culture cells, which often involve the rapid removal and subsequent return of amino acids to growth assays, have uniformly determined that all GATOR2 complex components are required for the activation of TORC1. In contrast, our whole-animal studies in Drosophila defined unique tissue-specific requirements for several individual GATOR2 subunits, including Mio, Seh1, and Wdr24. We determined that Wdr59, originally assigned to the GATOR2 complex based on studies performed in tissue culture cells, unexpectedly has a dual function in TORC1 regulation in Drosophila. We found that, in the ovary and the eye imaginal-disc brain complex, Wdr59 inhibits TORC1 activity by opposing the GATOR2–dependent inhibition of GATOR1. Specifically, GATOR2 attenuates the binding of GATOR1 to the RagA component of the Rag GTPase. Conversely, in the Drosophila fat body, Wdr59 promotes the accumulation of the GATOR2 components Mio and Wdr24 and is required for TORC1 activation. Similarly, in mammalian HeLa cells, WDR59 prevents the proteolytic destruction of these GATOR2 proteins. Consistent with reduced levels of the TORC1–activating GATOR2 complex, Wdr59–knockout HeLa cells have reduced TORC1 activity, which is restored, along with GATOR2 protein levels, upon proteasome inhibition. Taken together, our data support the model in which the Wdr59 component of the GATOR2 complex functions to promote or inhibit TORC1 activity, depending on cellular or metabolic context. Importantly, our work challenges recent models of GATOR2 function based on structural analysis, which posit that the GATOR2 complex requires all core subunits, including Wdr59, to form a functional complex. Our studies broaden the understanding of the GATOR–TORC1 signaling axis in metazoans and highlight the complexity of metabolic regulation in vivo. The GATOR–TORC1 signaling pathway is frequently cited as a potential target of pharmaceutical intervention because of its role in cancer, neurodegeneration, and epilepsy. Thus, it is essential to have a full mechanistic understanding of the in vivo function of the GATOR complex in the regulation of TORC1 signaling and growth.
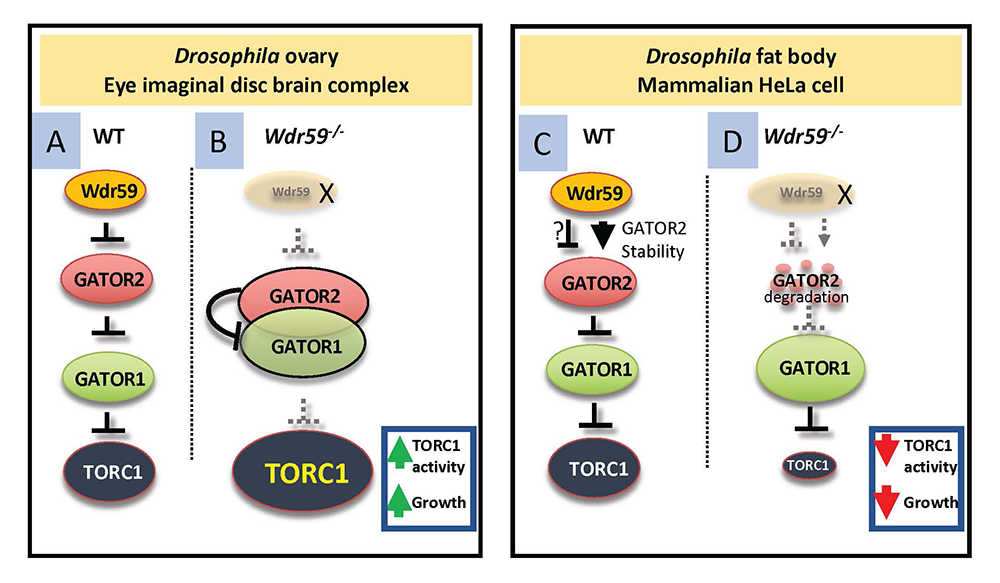
Figure 2. Model for the dual role of Wdr59 in TORC1 regulation
A. In wild-type animals, Wdr59 inhibits TORC1 activity upstream of the GATOR2 complex in the ovary and eye imaginal-disc brain complex.
B. In wdr59 mutants, GATOR2 increases its association with GATOR1, further inhibiting GATOR1 activity, and allowing for the increased activation of TORC1.
C. In the fat body of Drosophila and in mammalian HeLa cells, Wdr59 protects GATOR2 components from degradation by the proteasome.
D. In the absence of Wdr59 from the Drosophila fat body or from HeLa cells, GATOR2 components are destroyed by the proteasome, resulting in de-repression of GATOR1, increased interaction of GATOR1 with RagA, and concomitant reduction in TORC1 activity. Note that the GATOR1 complex regulates TORC1 activity by acting as a GAP (GTPase–activating protein) for the RagA component of the GTPase, which functions to activate TORC1.
Figure 2. Model for the dual role of Wdr59 in TORC1 regulation
A. In wild-type animals, Wdr59 inhibits TORC1 activity upstream of the GATOR2 complex in the ovary and eye imaginal-disc brain complex.
B. In wdr59 mutants, GATOR2 increases its association with GATOR1, further inhibiting GATOR1 activity, and allowing for the increased activation of TORC1.
C. In the fat body of Drosophila and in mammalian HeLa cells, Wdr59 protects GATOR2 components from degradation by the proteasome.
D. In the absence of Wdr59 from the Drosophila fat body or from HeLa cells, GATOR2 components are destroyed by the proteasome, resulting in de-repression of GATOR1, increased interaction of GATOR1 with RagA, and concomitant reduction in TORC1 activity. Note that the GATOR1 complex regulates TORC1 activity by acting as a GAP (GTPase–activating protein) for the RagA component of the GTPase, which functions to activate TORC1.
Publications
- The GATOR2 complex maintains lysosomal-autophagic function by inhibiting the protein degradation of MiT/TFEs. Mol Cell 2024 84:727–743
- Unveiling GATOR2 function: novel insights from Drosophila research. Cells 2024 13(21):1795
- Mechanical stimulation from the surrounding tissue activates mitochondrial energy 1 metabolism in differentiating germ cells. Dev Cell 23 58:2249–2260
- Wdr59 promotes or inhibits TORC1 activity depending on cellular context. Proc Natl Acad Sci USA 2022 120:e2212330120
Collaborators
- Ryan Dale, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
- Thomas Gonatopoulos-Pournatzis, PhD, Functional Transciptomics Section, NCI, Frederick, MD
- Marston Linehann, MD, Center for Cancer Research, NCI, Bethesda, MD
- Hong Xu, PhD, Laboratory of Molecular Genetics, NHLBI, Bethesda, MD
- Yingbiao Zhang, PhD, Institute for Translational Medicine, The Affiliated Hospital of Qingdao University, Qingdao, China
Contact
For more information, email mlilly@helix.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/lilly.