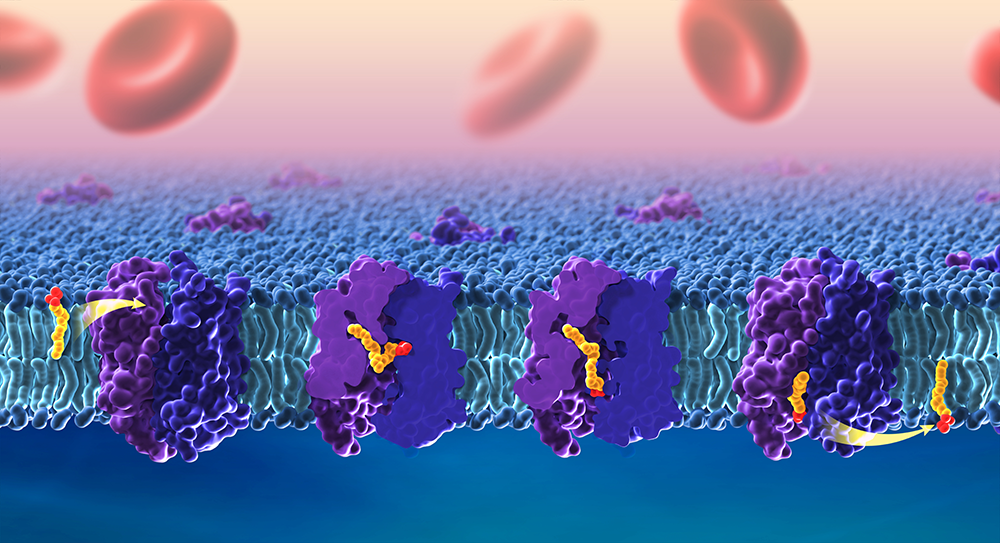
High-Resolution Structural Biology of Membrane Protein Complexes in Their Native Environment
- Doreen Matthies,
PhD, Head, Unit on Structural Biology - Fei Zhou, PhD, Scientist
- Peter R. Johnson, Cryo-Electron Microscopy/Light Microscopy Specialist
- Madolyn P. Britt, PhD, Postdoctoral Fellow
- Nicholas D. Clark, PhD, Postdoctoral Fellow
- Zaid K. Madni, PhD, Postdoctoral Fellow
- Louis Tung Faat Lai, PhD, Postdoctoral Fellow
- Elissa J. Moller, BS, Graduate Student
- Patrick E. O'Reilly, Postbaccalaureate Fellow
- Isadora Rocha De Abreu, BS, Postbaccalaureate Fellow
- Jasmin D. Wu, Student Intramural Research and Training Awardee

We are interested in the structure and function of membrane protein complexes in their native lipid-membrane environment to understand their mechanism, the influence of their immediate surrounding, and how these affect human health and disease. A cell contains many different lipid membranes with various lipid contents and distributions, which are very important for a membrane’s morphology and function. However, very little is understood about how the various micro-environments are formed and maintained and how they influence the structure and function of membrane proteins. Studying membrane protein complexes in their native biological membrane is therefore required.
We use a combination of molecular biology, biochemistry, and biophysical methods to study molecular transport across membranes, with a focus on how the immediate native environment influences the structure and function of membrane proteins, but also how proteins and lipids shape and functionalize a lipid membrane.
Cryo-electron microscopy (Cryo-EM) is one of the main structural biology methods of our lab. Using single-particle Cryo-EM, we solve high-resolution structures of membrane proteins in artificial environments such as in detergent micelles and lipid nano-discs. We are extending this approach to studying membrane proteins in their native environment, using native lipid nano-discs, membrane fractions in the form of vesicles, and intact cells and tissues, and we are using a combination of correlative light and electron-microscopy techniques, including cryo-fluorescent microscopy, cryo-focused ion beam-scanning electron microscopy (Cryo-FIBSEM), Cryo-EM, and cryo-electron tomography (Cryo-ET).
Figure 1. Three-dimensional cryo-EM structure of the human inner mitochondrial membrane magnesium channel MRS2 [Reference 5]
The channel is shown from the side and as a cross-section embedded in a lipid bilayer. The intermembrane space of the mitochondria is shaded blue, and the mitochondrial matrix is shaded purple. Magnesium ions are small green spheres. Credit: Ethan Tyler, NIH Medical Arts; Louis Tung Faat Lai, Matthies lab.
Figure 1. Three-dimensional cryo-EM structure of the human inner mitochondrial membrane magnesium channel MRS2 [Reference 5]
The channel is shown from the side and as a cross-section embedded in a lipid bilayer. The intermembrane space of the mitochondria is shaded blue, and the mitochondrial matrix is shaded purple. Magnesium ions are small green spheres. Credit: Ethan Tyler, NIH Medical Arts; Louis Tung Faat Lai, Matthies lab.
Structure and function of magnesium channels and transporters
Magnesium (Mg2+) is the most abundant divalent cation inside cells, with an average Mg2+ concentration of about 20 mM, most of it bound to proteins and ATP. Magnesium plays an essential role in cellular physiology, acting as a cofactor for more than 600 enzymes, including protein kinases, ATPases, exonucleases, and other nucleotide-related enzymes. Deficiency in Mg2+ is associated with diseases such as muscular dysfunction, bone wasting, immunodeficiency, cardiac syndromes, and neuronal disorders. The bacterial magnesium channel CorA is a homo-pentameric channel, which forms a symmetric closed state at normal to high concentrations of magnesium, with magnesium-binding sites between protomers as well as near the membrane pore. At low magnesium concentrations, the channel undergoes an asymmetric opening, which is likely to be caused by the destabilization of protomer interactions when magnesium ions dissociate from their binding site. The lab has expanded the research on magnesium channels, including eukaryotic magnesium channels. We recently resolved high-resolution structures of the human inner mitochondrial-membrane magnesium channel MRS2 and investigated its function and regulation [Reference 5]. To further investigate the structure and mechanism of these channels, structural studies on synthetic and native nano-discs, as well as in liposomes, are ongoing, and promising drug candidates are being tested on a variety of bacterial as well as eukaryotic channels.
Besides studying magnesium channels, which allow magnesium ions to diffuse rapidly down their electrochemical gradient across the lipid bilayer, we also study magnesium transporters, which actively transport magnesium ions against their gradient with the help of ATP hydrolysis. P-type ATPase transporters are required for growth when Mg2+ is limiting or during bacterial pathogenesis. We recently resolved the first structure of the Mg2+ transporter MgtA by single-particle cryo-EM. Using mild membrane extraction, we obtained a high-resolution structure of a homo-dimeric form, the first for a P-type ATPase, which typically are structurally known as monomeric units. We used a combination of cryo-EM, molecular dynamics simulations, hydrogen-deuterium exchange mass spectrometry, mutagenesis, and functional studies to characterize the magnesium transporter MgtA from E. coli, and we are extending these studies to magnesium transporters from pathogenic bacteria, as well as to eukaryotic homologs, to understand their mechanism of transport and regulation.
Uptake of essential omega-3 fatty acids
Omega-3 fatty acids are considered essential because the human body cannot make their own and must obtain them through foods, such as algae, wild fish, walnuts, and flaxseeds. DHA in particular is enriched in the brain and is important for a healthy nervous system. Breastmilk and most infant formulas contain DHA, and deficiencies in this fatty acid have been linked to problems with learning and memory. To reach the brain, omega-3 fatty acids must pass through the blood-brain barrier via the lipid transporter MFSD2A. The transporter is essential for normal brain development and is targeted by the Zika virus, which can cause microcephaly (i.e., a smaller-than-expected head) when infections occur during pregnancy. Despite its importance, we did not know precisely how MFSD2A transports DHA and other omega-3 fatty acids. In a new study, our team provided five cryo-electron microscopy structures of zebrafish Mfsd2a, which is structurally similar to its human counterpart. The snapshots are the first to detail precisely how fatty acids move across the cell membrane. We identified three compartments in Mfsd2a that suggest distinct steps required to move and flip fatty acids through the transporter, as opposed to movement through a linear tunnel or along the surface of the protein complex. Our findings [Reference 3] promise to improve understanding of lipid transport across the blood-brain barrier and of disruptions in this process, which can lead to birth defects or neurological conditions. The model also enables researchers to design drug molecules that are capable of directly reaching the brain. Overall, the findings provide key information on how MFSD2A transports omega-3 fatty acids into the brain, findings that may enable researchers to optimize drug delivery via this route. The study also provides foundational knowledge for understanding how other members of this transporter family, called the major facilitator superfamily (MFS), regulate important cellular functions.
Figure 2. Cryo-EM of the zebrafish lipid transporter Mfsd2a reveals stepwise lipid translocation.
The brain acquires essential omega-3 fatty acids through transport across the blood-brain barrier via the lipid transporter Mfsd2a. Cryo-EM of zebrafish Mfsd2a revealed different snapshots of its translocation pathway, detailing how lipids are flipped from the outer leaflet of the lipid bilayer and released in the inner leaflet. Credit: Ethan Tyler, NIH Medical Arts; Louis Lai, Matthies lab, in collaboration with the Gonen lab.
Figure 2. Cryo-EM of the zebrafish lipid transporter Mfsd2a reveals stepwise lipid translocation.
The brain acquires essential omega-3 fatty acids through transport across the blood-brain barrier via the lipid transporter Mfsd2a. Cryo-EM of zebrafish Mfsd2a revealed different snapshots of its translocation pathway, detailing how lipids are flipped from the outer leaflet of the lipid bilayer and released in the inner leaflet. Credit: Ethan Tyler, NIH Medical Arts; Louis Lai, Matthies lab, in collaboration with the Gonen lab.
Collaborations
Our collaborations involve structural and computational studies on a variety of membrane-protein complexes, including transporters, channels, and receptors, virus-like particles (VLP), SARS-CoV-2 accessory membrane proteins, extracellular vesicles, and lipid transport across cells, as well as on novel detergents and polymers, designed to gently extract membrane-protein complexes from their native lipid environment, for high-resolution structural studies.
Publications
- Unique aggregation of retroviral particles pseudotyped with the delta variant SARS-CoV-2 spike protein. Viruses 2022 14:1024
- Autolysosomal exocytosis of lipids protect neurons from ferroptosis. J Cell Biol 2023 222:e202207130
- Lipid flipping in the omega-3 fatty-acid transporter. Nat Commun 2023 14:2571
- Structure-function analyses reveal key molecular determinants of HIV-1 CRF01_AE resistance to the entry inhibitor temsavir. Nat Commun 2023 14:6710
- Cryo-EM structures of human magnesium channel MRS2 reveal gating and regulatory mechanisms. Nat Commun 2023 14:7207
Collaborators
- Tamir Gonen, PhD, Laboratory of Molecular Electron Microscopy, UCLA, Los Angeles, CA
- Rick K. Huang, PhD, NIH Intramural Research CryoEM Consortium, NCI, Bethesda, MD
- Maria S. Ioannou, PhD, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Canada
- Marzena E. Pazgier, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Alexander J. Sodt, PhD, Unit on Membrane Chemical Physics, NICHD, Bethesda, MD
- Gisela Storz, PhD, Section on Environmental Gene Regulation, NICHD, Bethesda, MD
- Sergei I. Sukharev, PhD, University of Maryland, College Park, MD
- Joshua J. Zimmerberg, MD, PhD, Section on Integrative Biophysics, NICHD, Bethesda, MD
- Manuela Zoonens, PhD, Institut de Biologie Physico-Chimique, Centre National de la Recherche Scientifique, Paris, France
Contact
For more information, email doreen.matthies@nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/matthies.


![Figure 1. Three-dimensional cryo-EM structure of the human inner mitochondrial membrane magnesium channel MRS2 [Reference 5]](images/matthies_2024_fig1.png)