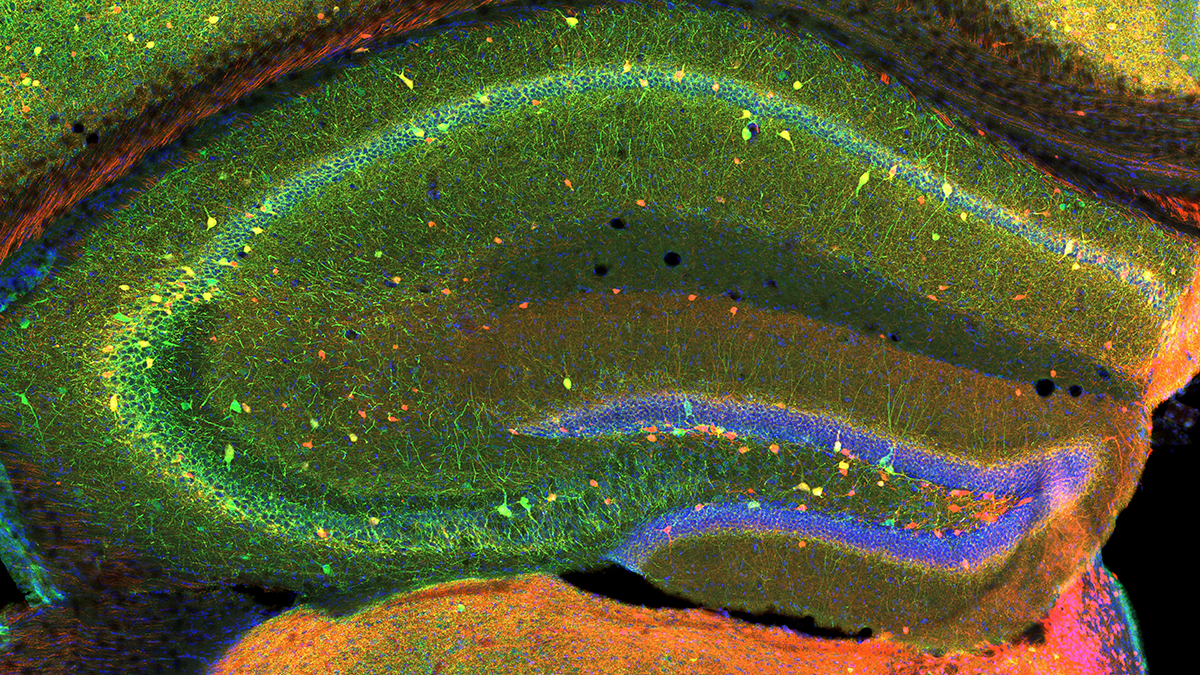
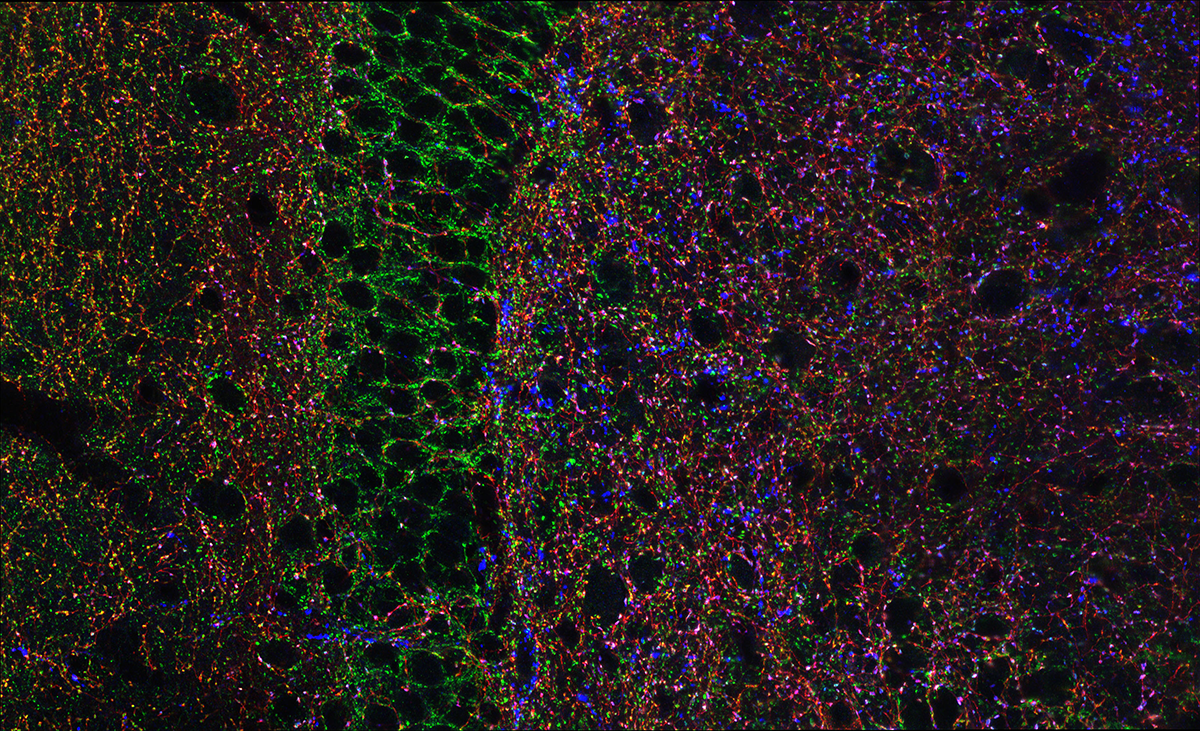
Hippocampal Interneurons and Their Role in the Control of Network Excitability
- Chris J. McBain,
PhD, Head, Section on Cellular and Synaptic Physiology - Ramesh Chittajullu, PhD, Staff Scientist
- Edra London, PhD, Staff Scientist
- Kenneth Pelkey, PhD, Staff Scientist
- Adam Caccavano, PhD, Postdoctoral Fellow
- Lauren Hewitt, PhD, Postdoctoral Fellow
- June Hoan Kim, PhD, Postdoctoral Fellow
- Geoffrey Vargish, PhD, Postdoctoral Fellow
- Steven Hunt, BS, Biologist
- Xiaoqing Yuan, MSc, Biologist
- Erica Lesko, BS, Postbaccalaureate Fellow
- Nadiya McLean, BS, Postbaccalaureate Fellow
- Anna Vlachos, BSc, Postbaccalaureate Fellow

Cortical and hippocampal GABAergic inhibitory interneurons (INs) are ‘tailor-made’ to control cellular and network excitability by providing synaptic and extrasynaptic input to their downstream targets via GABAA and GABAB receptors. The axons of this diverse cell population make mostly local, short-range projections, although some subpopulations project their axons over considerable distances, and release the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) onto a variety of targets. Distinct cohorts of INs regulate sub- and supra-threshold intrinsic conductances, regulate Na+– and Ca2+–dependent action-potential generation, modulate synaptic transmission and plasticity, and pace both local and long-range, large-scale synchronous oscillatory activity. An increasing appreciation of the roles played by INs in several neural-circuit disorders, such as epilepsy, stroke, Alzheimer’s disease, and schizophrenia, has seen this important cell type take center stage in cortical circuit research. With almost 30 years of interest in INs, the main objectives of the lab have been to understand: (1) the developmental trajectories taken by specific cohorts of INs as they populate the nascent hippocampus and cortex; (2) how ionic and synaptic mechanisms regulate the activity of both local-circuit GABAergic INs and principal neurons (PN) at the level of small, well defined networks; and (3) how perturbations in their function alter the cortical network in several neural-circuit disorders. To this end, we use a variety of electrophysiological, imaging, optogenetic, immunohistochemical, biochemical, molecular, and genetic approaches with both wild-type and transgenic animals.
Background and significance
In the hippocampus (HPC), GABAergic local circuit inhibitory INs account for about 10–15% of the total neuronal cell population. Despite being in the minority, this diverse neuronal population serves as a major determinant of all aspects of cortical circuit function and regulation. Within the HPC, the cell bodies of INs are scattered across all major subfields, and the positioning of their somatodendritic arbors allows integration of input from a number of intrinsic and extrinsic afferent inputs. The axons of many IN subtypes largely remain local to the subfield housing their soma and dendrites; however, many form long-range projections that extend beyond their original location to ramify within both cortical and subcortical structures. Their axons target well defined narrow postsynaptic domains (i.e., soma and proximal dendrites) or can provide widespread input to large portions of target cell dendrites. This innervation of different postsynaptic cellular compartments ensures that virtually all domains of their principal neuron targets receive extensive coverage and, importantly, underscores that IN subtypes perform distinct roles in the hippocampal circuit. INs are primarily providers of inhibitory GABAergic synaptic input, a physiological role that utilizes Cl– influx or K+ efflux via cognate GABAA– or GABAB–receptor activation respectively, to transiently hyperpolarize or shunt the cell membrane away from action-potential threshold. These interneurons play major roles in not only the regulation of single-cell excitability, but provide well timed inhibitory input that dictates the temporal window for synaptic excitation, and subsequent action-potential initiation, thus shaping the timing of afferent and efferent information flow. In addition, they harness and synchronize both local and distributed cortical circuits to facilitate oscillatory activity across broad frequency domains. Indeed, several developmentally regulated neural circuit disorders, such as epilepsy, schizophrenia, and autism, are likely associated with deficits in the numbers and function of distinct IN cohorts. For all these reasons, INs have recently become the intense focus of investigators drawn from a wide variety of backgrounds.
Our current research has focused on three main aspects of IN function: (1) we continue to study glutamatergic and GABAergic synaptic transmission made onto and from INs and their downstream targets, within the hippocampal and cortical formations; (2) we continue to capitalize on and expand our research using genetic and viral approaches to examine the development of specific cohorts of medial- and caudal-ganglionic eminence–derived INs and their roles in both nascent and mature circuits; and (3) as part of a multi-institute consortium, we have expanded our studies to consider evolutionary conservation or diversity of principal-neuron and interneuron function, using tissue derived from a number of species and, in collaboration with NINDS neurosurgery, surgically resected human hippocampal and cortical tissue. This multi-parametric research approach to circuit development and function has been extremely fruitful and is a perfect example of why our research strategy is well suited for the intramural environment. Having the flexibility to pursue this line of research would not have been possible without the support and collaborative nature of the NIH intramural program.
Divergent opioid-mediated suppression of inhibition between hippocampus and neocortex across species and development
Opioid receptors within the CNS regulate pain sensation and mood and are key targets for drugs of abuse. Within the adult rodent hippocampus (HPC), μ-opioid receptor agonists suppress inhibitory, parvalbumin-expressing interneurons (PV-INs), thus disinhibiting the circuit. However, it is uncertain whether this disinhibitory motif is conserved in other cortical regions, species, or across development. We observed that PV-IN–mediated inhibition is robustly suppressed by opioids in the HPC proper but not the neocortex in mice and nonhuman primates, with spontaneous inhibitory tone in resected human tissue also following a consistent dichotomy. This hippocampal disinhibitory motif was established in early development, when PV-INs and opioids were found to regulate primordial network rhythmogenesis. Acute opioid-mediated modulation was partially occluded with morphine pretreatment, with implications for the effects of opioids on hippocampal network activity important for learning and memory. Together, these findings demonstrate that PV-INs exhibit a divergence in opioid sensitivity across brain regions that is remarkably conserved across evolution, and they highlight the under-appreciated role of opioids, acting through immature PV-INs, in shaping hippocampal development.
GluN1/GluN3a excitatory glycine receptor (eGlyR)–mediated tonic currents in hippocampal interneurons control network dynamics throughout development.
The NMDA receptor (N-methyl-D-aspartate receptor, NMDAR) plays a crucial role in learning and memory processes within the brain. As a type of glutamate receptor, the NMDA receptor is involved in the synaptic plasticity that underlies the formation and strengthening of neural connections, so called long-term potentiation, a mechanism believed to be a cellular basis for learning and memory. NMDARs are composed of a combination GluN1, GluN2, GluN3 subunits, and the most common NMDAR types are GluN1/GluN2 diheteromeric receptors. Recently, GluN1/GluN3a excitatory glycine receptor (eGlyR)–mediated currents were reported to tonically influence membrane potential and excitability of neurons in the cortex, medial habenula, and amygdala. These NMDARs are distinct from those expressing GluN2–containing conventional NMDARs, in that they are voltage-independent and gated solely by glycine. Within the HPC, amongst various neuronal types, somatostatin-containing inhibitory interneurons (SST-INs) express remarkably high levels of GRIN3A, a gene encoding GluN3a, throughout development; several subtypes of NPY–expressing neurogliaform cells also express GRIN3A. We determined that the excitability of SST-/NPY-IN is strongly influenced by these eGlyRs from early postnatal to adult ages. We found that eGlyR–mediated inward currents in NPY-INs are a major contributor to the GABAergic tone onto pyramidal neurons in the developing HPC, with major consequences for the generation of giant depolarizing potential (GDP). GDPs are rhythmically occurring waves across the neural network that exist between birth to about 10 postnatal days in the HPC and cortex, driving synaptic maturation through activity-dependent plasticity. In the mature HPC, eGlyR–mediated currents in SST-IN eGlyRs also regulate sharp wave ripples (SWRs), network oscillations associated with memory consolidation. Our data are consistent with SST-IN eGlyRs being tonically engaged by endogenous glycine rather than by phasic input from afferents, consistent with prior observations that conventional NMDAR co-agonist binding sites are tonically occupied. Collectively, the data suggest that eGlyRs influence SST-/NPY-IN excitability to regulate synchronized network rhythms associated with circuit and memory formation, yielding novel insight into the physiological roles of the notoriously enigmatic GluN3A subunit. In addition, we obtained preliminary data showing that SST-IN excitability is increased by eGlyR activation in the non-human primate HPC, thus influencing SWRs frequency.
A novel enhancer-AAV approach selectively targeting dentate granule cells
The mammalian brain contains a diverse array of cell types, including dozens of neuronal subtypes with distinct anatomical and functional characteristics. The brain leverages these neuron-type specializations to perform diverse circuit operations and thus execute different behaviors properly. Through the use of Cre lines, access to specific neuron types has improved over past decades. Despite their extraordinary utility, development and cross-breeding of Cre lines is time-consuming and expensive, presenting a significant hurdle for investigators. Furthermore, cell-based therapeutics developed in Cre mice are not clinically translatable. Recently, several adeno-associated virus (AAV) vectors utilizing neuron type–specific regulatory transcriptional sequences (enhancer-AAVs) were developed, which overcome these limitations. Using a publicly available RNA sequencing (RNA-Seq) dataset, we evaluated the potential of several candidate enhancers for neuron type–specific targeting in the HPC. We demonstrated that a previously identified enhancer–AAV selectively targets dentate granule cells over other excitatory neuron types in the HPC of wild-type adult mice. By comparing the expression of scRE–regulated genes with an established RNA sequencing map of the HPC, we found that a target gene regulated by the scRE4 enhancer showed higher expression in dorsal DG granule cells than in other excitatory neuron types. When incorporated into an enhancer AAV to drive YFP expression (yellow fluorescent protein, enables visualization of gene expression and location), we observed abundant and highly selective labeling of dentate gyrus (DG) granule cells in the dorsal HPC. Expression was present in all subcellular compartments of the granule cells, and importantly, we detected no measurable expression in other neighboring excitatory cell types in the CA3 or hilus regions of the HPC. Further, we demonstrated that systemic delivery of this enhancer-AAV to drive Cre expression will allow for genetic or activity manipulation of DG granule cells across mouse models. Together, we identified a viral approach to selectively target hippocampal granule cells, which allows specific presynaptic (mossy fiber boutons and axons) genetic modulation and may thus, in the future, provide a tool for targeted clinical interventions at the mossy fiber–CA3 synapse.
Additional Funding
- Adam Caccavano was partially funded by a Center for Compulsive Disorder Fellowship award.
Publications
- Evolutionary conservation of hippocampal mossy fiber synapse properties. Neuron 2023 111:3802–3818
- NMDARs drive the expression of neuropsychiatric disorder risk genes within GABAergic interneuron subtypes in the juvenile brain. Front Mol Neurosci 2021 14:712609
- A novel enhancer-AAV approach selectively targeting dentate granule cells. Cell Rep Methods 2024 4(1):100684
Collaborators
- Bruno Averbeck, PhD, Section on Learning and Decision Making, NIMH, Bethesda, MD
- Jordan Dimidschstein, PhD, The Broad Institute, Cambridge, MA
- Gordon Fishell, PhD, The Broad Institute, Cambridge, MA
- Timothy J. Petros, PhD, Unit on Cellular and Molecular Neurodevelopment, NICHD, Bethesda, MD
- Mathew Rowan, PhD, Emory University, Atlanta, GA
- Kareem Zaghloul, MD, PhD, Functional and Restorative Neurosurgery Unit, NINDS, Bethesda, MD
Contact
For more information, email mcbainc@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/mcbain.