Rare Genetic Disorders of Cholesterol Homeostasis and Lysosomal Diseases
- Forbes D. Porter,
MD, PhD, Head, Section on Molecular Dysmorphology - Niamh X. Cawley, PhD, Staff Scientist
- Cristin Davidson, PhD, Staff Scientist
- Derek Alexander, MPH, Protocol Coordinator
- Desiree Labor, MSN, Research Nurse Practitioner
- Antony Cougnoux, PhD, Collaborator
- Emelin Hernandez, Animal Technician
- Sarita Kumari, PhD, Visiting Fellow
- Shikha Salhotra, PhD, Visiting Fellow
- Khushboo Singhal, PhD, Visiting Fellow
- Carolina Alvarez, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Susan Gembic, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Katerina Melnyk, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Hibaaq Mohamed, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Avani Mylvara, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Aishwarya Selvaraman, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Christian White, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Andrea Munoz, BS, Special Volunteer

We study the molecular, biochemical, and cellular processes that underlie genetic disorders resulting from impaired cholesterol homeostasis and from lysosomal dysfunction. The disorders include malformation/cognitive impairment syndromes resulting from inborn errors of cholesterol synthesis and neurodegenerative disorders resulting from impaired intracellular cholesterol and lipid transport. Human malformation syndromes attributable to inborn errors of cholesterol synthesis include the Smith-Lemli-Opitz syndrome (SLOS), lathosterolosis, desmosterolosis, X-linked dominant chondrodysplasia punctata type 2 (CDPX2), and the CHILD syndrome. We also study Niemann-Pick disease type C (NPC), as well as Juvenile Batten disease caused by pathogenic variants in the gene CLN3 (CLN3 disease). Both NPC and CLN3 disease are lysosomal diseases that result in progressive neurodegeneration. Our research group uses basic, translational, and clinical research approaches with the ultimate goal of developing and testing therapeutic interventions for these rare genetic disorders. Our basic research uses induced pluripotent stem cells (iPSC)–derived neuronal and mouse models of these genetic disorders to understand the biochemical, molecular, cellular, and developmental processes that underlie the birth defects and clinical problems encountered in affected patients. Our clinical research focuses on translating basic findings to the clinic. Natural history trials of SLOS and NPC1 are ongoing. We have large cross-sectional and longitudinal collections of biomaterial from individuals whose disease course and phenotype are known, and such samples can be used for both for biomarker discovery and validation. Therapeutic trials have been conducted for SLOS, CLN3, and NPC1. Also, in collaboration with NCATS (the National Center for Advancing Translational Sciences), our research group has been involved in a multicenter trial of creatine transporter deficiency.
Section on Molecular Dysmorphology Research Team
Top: Khushboo Singhal, Sarita Kumari, Niamh Cawley, Emelin Hernandez, Aileen Barnes, Cameron Padilla, Garrett Bowman, Elizabeth Davis (MGC), Aiden Borruso, Antony Cougnoux, Susan Gembic, Shikha Salhotra, Cristin Davidson, Carolina Alvarez
Bottom: Derek Alexander, Desiree Labor, Cameron Padilla, Aiden Garrett Bowman, Samar Rahhal
Section on Molecular Dysmorphology Research Team
Top: Khushboo Singhal, Sarita Kumari, Niamh Cawley, Emelin Hernandez, Aileen Barnes, Cameron Padilla, Garrett Bowman, Elizabeth Davis (MGC), Aiden Borruso, Antony Cougnoux, Susan Gembic, Shikha Salhotra, Cristin Davidson, Carolina Alvarez
Bottom: Derek Alexander, Desiree Labor, Cameron Padilla, Aiden Garrett Bowman, Samar Rahhal
Inborn errors of cholesterol synthesis
Smith-Lemli-Opitz syndrome (SLOS)
SLOS is an autosomal recessive, multiple-malformation syndrome characterized by dysmorphic facial features, cognitive impairment, hypotonia, poor growth, and various structural anomalies of heart, lungs, brain, limbs, gastrointestinal tract, and genitalia. The SLOS phenotype is extremely variable. At the severe end of the phenotypic spectrum, infants often die as result of multiple major malformations, while mild SLOS combines minor physical malformations with behavioral and learning problems. The syndrome is the result of an inborn error of cholesterol biosynthesis that blocks the conversion of 7-dehydrocholesterol (7-DHC) to cholesterol.
Our laboratory initially cloned the human 3beta-hydroxysterol delta7-reductase gene (DHCR7) and demonstrated mutations of the gene in SLOS patients. Together with others, we have so far identified over 100 mutations in DHCR7. We also used gene targeting in murine embryonic stem cells to produce several SLOS mouse models, including a null deletion and a hypomorphic point mutation. Mouse pups homozygous for the null mutation (Dhcr7delta3–5/delta3–5) exhibit variable craniofacial anomalies, are growth-retarded, appear weak, and die during the first day of life because they fail to feed. Thus, we were not able to use them to study postnatal brain development, myelination, or behavior, or to test therapeutic interventions. For this reason, we developed a missense allele (Dhcr7T93M). The T93M mutation is the second most common mutation found in SLOS patients. Dhcr7T93M/T93M and Dhcr7T93M/delta3–5 mice are viable and demonstrate SLOS with a gradient of biochemical severity (Dhcr7delta3–5/delta3–5 greater than Dhcr7T93M/delta3–5 and greater than Dhcr793M/T93M). We used Dhcr7T93M/delta3–5 mice to test the efficacy of therapeutic interventions on tissue sterol profiles. As expected, dietary cholesterol therapy improved the sterol composition in peripheral tissues but not in the central nervous system. Treatment of mice with the statin simvastatin improved the biochemical defect in both peripheral and central nervous system tissue, suggesting that simvastatin therapy may be used to treat some of the behavioral and learning problems in children with SLOS. Most recently, we developed a zebrafish model for SLOS that will allow us to study the impact of aberrant cholesterol synthesis on behavior. Characterization of iPSCs from SLOS patients demonstrated a defect in neurogenesis, which results from inhibition of Wnt signaling owing to a toxic effect of 7-DHC.
We are conducting a longitudinal Natural History trial on SLOS. Given that SLOS patients have a cholesterol deficiency, they may be treated with dietary cholesterol supplementation. To date, we have evaluated 164 individuals with SLOS. This is the largest well characterized cohort in the world. The studies are now focused on defining the phenotype of older adolescents and young adults with SLOS. We are also working to identify cerebrospinal-fluid biomarkers that may provide insight into neurological dysfunction.
One reason for studying rare genetic disorders is to gain insight into more common disorders. Most patients with SLOS exhibit autistic characteristics. We are currently collaborating with other NIH and extramural groups to further evaluate this finding.
Niemann-Pick disease type C1
Niemann-Pick disease type C1 (NPC1) is a neurodegenerative disorder that results in ataxia and dementia. In view of the dementia, it has been referred to as childhood Alzheimer disease. The disorder is caused by a defect in intracellular lipid and cholesterol transport. Initially, as part of a bench-to-bedside award, we began a clinical protocol to identify and characterize biomarkers that could be used in a subsequent therapeutic trial. The project also received support from the Ara Parseghian Medical Research Foundation, Dana's Angels Research Trust, and Together Strong. We have enrolled 155 individuals with NPC1 in a longitudinal Natural History/Observational trial. The current goals of the trial are to identify: (1) biomarkers that can be used as tools to facilitate development and implementation of therapeutic trials; (2) clinical symptoms/signs that may be used as efficacy outcome measures in a therapeutic trial; (3) genetic modifiers.
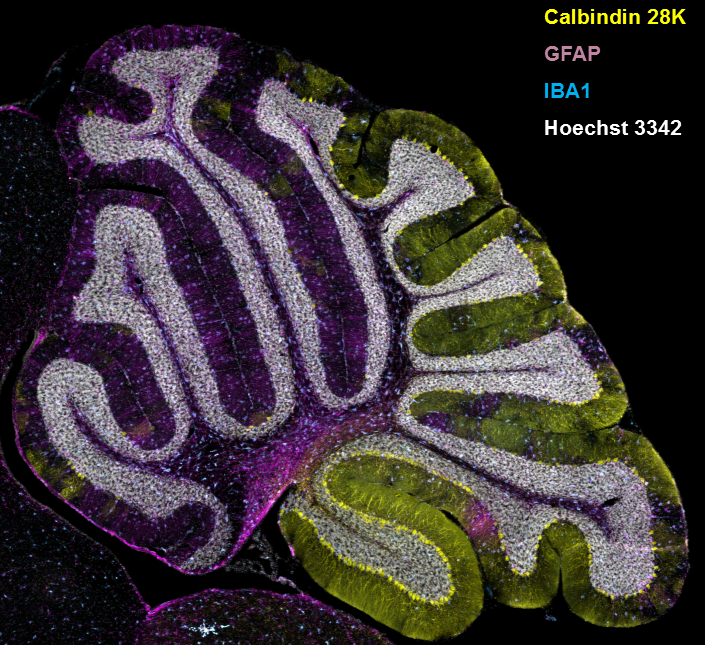
Figure 2. Gliosis in NPC1 mouse cerebellum
Immuno-staining of a sagittal section from the cerebellum of an NPC1–mutant mouse. Cerebellar Purkinje neurons are stained for calbindin 28K, and the expected loss of anterior Purkinje neurons is readily apparent. Expression of GFAP (glial fibrillary acidic protein) and IBA1 (ionized calcium binding adaptor molecule 1) are used to detect astrogliosis and microgliosis, respectively. Nuclei are stained with Hoechst 3342.
Figure 2. Gliosis in NPC1 mouse cerebellum
Immuno-staining of a sagittal section from the cerebellum of an NPC1–mutant mouse. Cerebellar Purkinje neurons are stained for calbindin 28K, and the expected loss of anterior Purkinje neurons is readily apparent. Expression of GFAP (glial fibrillary acidic protein) and IBA1 (ionized calcium binding adaptor molecule 1) are used to detect astrogliosis and microgliosis, respectively. Nuclei are stained with Hoechst 3342.
Currently, the average time from first symptom to diagnosis, the ‘diagnostic delay,’ in our cohort of NPC patients is on the order of four to five years. In collaboration with Daniel Ory, we found elevated levels of non-enzymatically produced oxysterols in NPC1 patients. Testing for oxysterols or bile-acid derivatives has now become a standard method of diagnosis, and they are a potential biomarker that may be used to follow therapeutic interventions. We are now involved in a collaboration to implement newborn screening for NPC.
In addition to our Natural History study, we completed a randomized, placebo-controlled, cross-over trial to investigate the safety and efficacy of N-acetyl cysteine (NAC) in NPC1. The goal was to determine whether NAC treatment would reduce oxidative stress and subsequently lower levels of the non-enzymatically produced oxysterols. We also tested the safety and efficacy of the histone deacetylase (HDAC) inhibitor vorinostat in adult NPC1 patients. In collaboration with the Therapeutics of Rare and Neglected Disease Program of NCATS, we completed a phase 1/2a therapeutic trial of lumbar intrathecal cyclodextrin (VTS-270, adrabetadex) therapy in NPC1. We participated in a multicenter, international phase 2b/3 of adrabetadex and investigated the safety and efficacy of combined intrathecal and intravenous adrabetadex. In collaboration with investigators at St. Louis Children's Hospital, we are studying the efficacy of cyclodextrin to ameliorate liver disease in infants with NPC. We are now collaborating with several groups in order to advance gene therapy.
To complement the clinical work, we have begun to apply molecular and proteomic approaches to both mouse and human biomaterials in order to identify biological pathways disrupted in NPC1. We identified several blood and CSF (cerebral spinal fluid) proteins and are in the process of validating the biomarkers as potential outcome measures to be used as tools in the development of therapeutic interventions. In collaboration with investigators from NHGRI and Scripps, we are obtaining genomic sequences on a large cohort of well phenotyped individuals with NPC1. Utilizing novel machine learning/artificial intelligence techniques, we are attempting to gain insight into genetic modifiers of the NPC1 phenotype.
Development of NPC–induced pluripotent stem cell lines that can be efficiently differentiated into neurons has permitted us to initiate a number of studies in collaboration with NCATS to find drugs/genes that reduce endolysosomal cholesterol storage in NPC1 neurons.
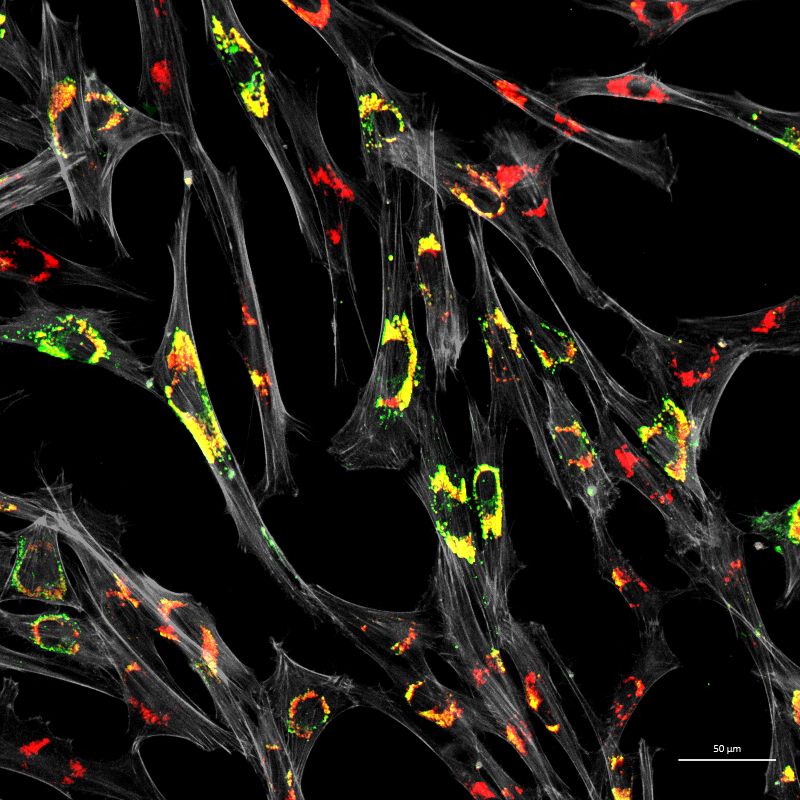
Figure 3. Accumulation of unesterified cholesterol in NPC1–patient fibroblasts
Human NPC1 fibroblasts were immuno-stained for Lamp1 (green) and stained with filipin (red); filipin stains unesterified cholesterol, which accumulates in the Lamp1–positive endolysosomal compartment. Cell structure was outlined by immuno-staining for actin (gray).
Figure 3. Accumulation of unesterified cholesterol in NPC1–patient fibroblasts
Human NPC1 fibroblasts were immuno-stained for Lamp1 (green) and stained with filipin (red); filipin stains unesterified cholesterol, which accumulates in the Lamp1–positive endolysosomal compartment. Cell structure was outlined by immuno-staining for actin (gray).
CLN3 disease
CLN3 disease (Juvenile Batten disease) is an autosomal recessive, progressive neurodegeneration arising from mutation of CLN3, the gene encoding the lysosomal/endosomal protein battenin. The function of the battenin is not known, but its absence leads to a lysosomal storage disorder. Children with CLN3 disease typically first lose vision, followed by progressive cognitive and motor impairment. Similar to the other disorders that we study, our goal is to conduct a natural history study in order to facilitate studies designed to understand the pathology underlying these disorders as well as to develop therapeutic interventions. A major effort in our laboratory is to identify biomarkers that provide insight into CLN3 pathology and facilitate therapeutic trials. A diagnostic metabolite, especially one that could be used in a newborn screen, would be a major advance in the field.
To complement our clinical research, we are also studying CLN3 in our laboratory. We have developed an induced pluripotent stem-cell line that can be efficiently induced to form CLN3–deficient neurons. The neurons are being used in genome-wide CRISPRi screens to identify genes that modify the CLN3 neuronal phenotype. The induced pluripotent stem cell (iPSC)–derived neurons will also be used to screen for potential therapeutic drugs.
The work on CLN3 disease is being transferred to An Dang Do, Head of the Unit on Cellular Stress in Development and Diseases, NICHD.
Creatine transport deficiency
In collaboration with NCATS, we completed a natural history trial of creatine transport deficiency (CTD). CTD is an X-linked disorder arising from mutation in SLC6A8 (which encodes solute carrier family 6 Member 8, a sodium- and chloride-dependent creatine transporter). Individuals with CTD manifest significant developmental delay and have frequent seizures. Our goals were to obtain detailed natural history data, establish a biorepository, find biomarkers, and identify potential clinical outcome measures in preparation for a therapeutic trial. A major clinical finding of this natural history trial was the detailed characterization of prolonged electrocardiographic QTc in many of the individuals with CTD, which has led to specific clinical recommendations.
Additional Funding
- Ara Parseghian Medical Research Fund, University of Notre Dame
- Together Strong NPC Foundation
- Mandos Health CRADA
- Amicus CRADA
- Beyond Batten Disease Foundation CRADA
- SOAR NPC
- FireFly Fund
- NICHD Scientific Director's Award
Publications
- Modeling Smith-Lemli-Opitz syndrome with induced pluripotent stem cells reveals a causal role for Wnt/beta-catenin defects in neuronal cholesterol synthesis phenotypes. Nat Med 2016 22:388–396
- Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: an ad-hoc analysis of a non-randomized, open-label, phase 1/2 trial. Lancet 2017 390(10104):1758–1768
- Single cell transcriptome analysis of Niemann-Pick disease, type C1 cerebella. Int J Mol Sci 2020 21(15):5368
- Association of miglustat with swallowing outcomes in Niemann-Pick disease, type C1. JAMA Neurol 2020 77(12):1564–1568
- Neurofilament light chain in cerebrospinal fluid as a novel biomarker in evaluating both clinical severity and therapeutic response in Niemann-Pick disease type C1. Genet Med 2023 25:100349
Collaborators
- William Balch, PhD, The Scripps Research Institute, La Jolla, CA
- Elizabeth Berry-Kravis, MD, PhD, Rush University Medical Center, Chicago, IL
- Juan Bonifacino, PhD, Section on Intracellular Protein Trafficking, NICHD, Bethesda, MD
- Ken Chang, PhD, Chemical Genomics Branch, NCATS, Rockville MD
- Stephanie Cologna, PhD, University of Illinois at Chicago, Chicago, IL
- Patricia Dickson, MD, St. Louis Children's Hospital, Washington University School of Medicine, St. Louis, MO
- Paul Helquist, PhD, University of Notre Dame, Notre Dame, IN
- Fang Liu, PhD, University of Notre Dame, Notre Dame, IN
- Judith Miller, PhD, Children's Hospital of Philadelphia, Philadelphia, PA
- Daniel Ory, MD, Institute of Clinical and Translational Sciences, Washington University, St. Louis, MO
- William J. Pavan, PhD, Genetic Disease Research Branch, NHGRI, Bethesda, MD
- Ninet Sinaii, PhD, Biostatistics and Clinical Epidemiology Service, NIH Clinical Center, Bethesda, MD
- Beth Solomon, MS, CCC-SLP, Rehabilitation Medicine Department, NIH Clinical Center, Bethesda, MD
- Audrey Thurm, PhD, Neurodevelopmental and Behavioral Phenotyping Service, NIMH, Bethesda, MD
- Charles H. Vite, DVM, PhD, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA
Contact
For more information, email fdporter@helix.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/porter.