Investigating Chromosome Structure and Function during Development
- Leah F. Rosin,
PhD, Stadtman Investigator and Head, Unit on Chromosome Dynamics - Christa Heryanto, PhD, Research Scientist and Lab Manager
- LingSze Lee, PhD, Postdoctoral Researcher
- Leif Benner, PhD, Postdoctoral Fellow
- Clio Hockens, BS, Postbaccalaureate Fellow
- Lorielle Raab, BS, Postbaccalaureate Fellow (Joint with NIDDK)
- Rose Runyan, BS, Postbaccalaureate Fellow
- Charli Wingfield, BS, Postbaccalaureate Fellow (Joint with NIDDK)

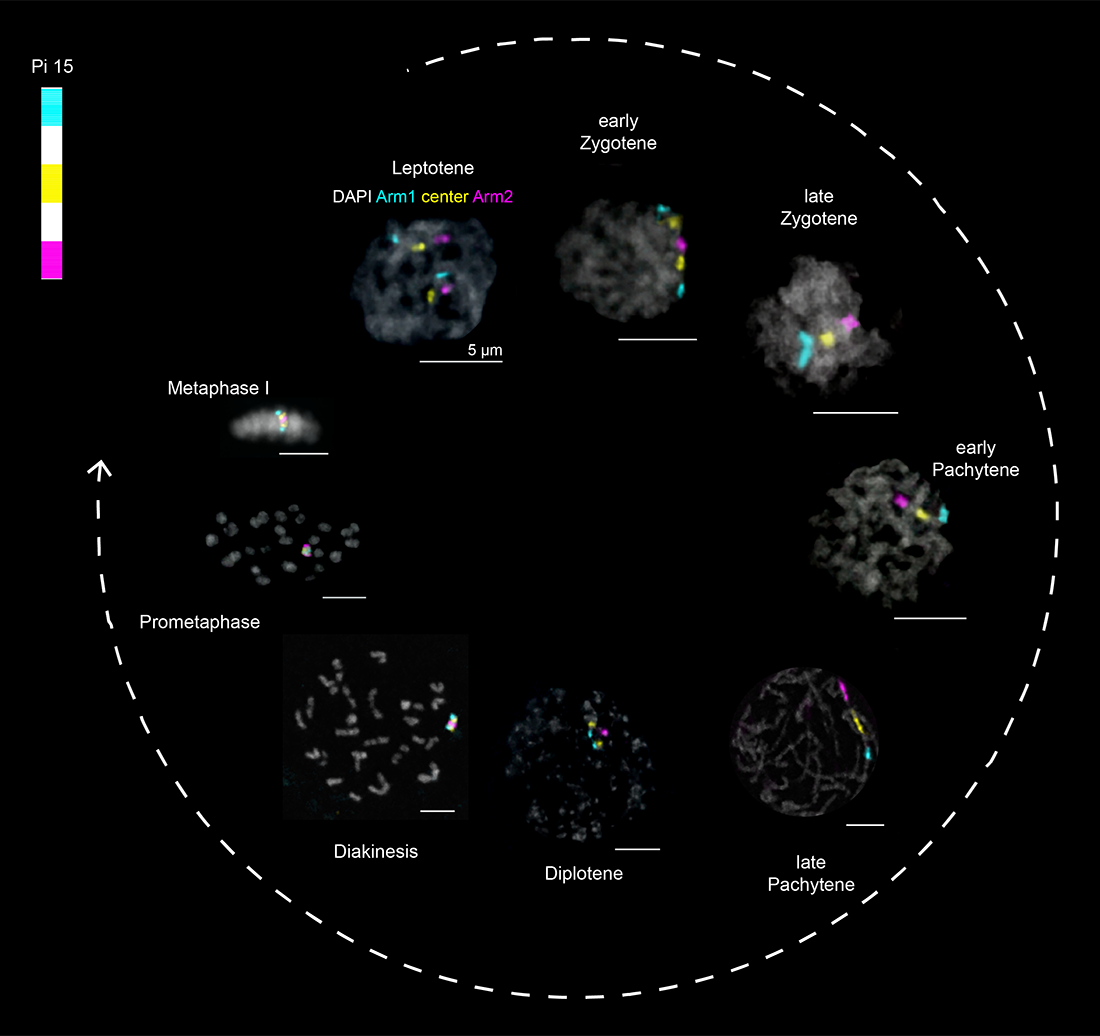
Precisely regulating the genome is absolutely essential for cell function. One extreme example of this is in the germline, where a subset of cells undergo a reductional division to become haploid and contribute to the next generation as egg or sperm cells. The chromatin dynamics and epigenetic changes involved in establishing and maintaining the germ cell lineage and accurately regulating the meiotic cell divisions are crucial for fertility and embryonic development across species. Still, many aspects of germline genome biology remain poorly understood, including: (1) the transcriptional reprogramming that happens during germ cell development; (2) the mechanisms regulating homolog pairing at the beginning of meiosis (Figure 1); (3) the designation of double-strand breaks to become sites of “crossing over” during meiotic recombination; and (4) kinetochore assembly and chromosome segregation during meiosis I and II.
Understanding these central aspects of chromosome biology will not only provide insights into how disruption of these processes can result in genome instability and developmental disorders in offspring but will provide new avenues for potential therapeutic targets to treat infertility.
To study the germline genome, our lab employs a combination of the Oligopaint fluorescence in situ hybridization (FISH) technology (Figure 2), live imaging, and high-throughput genomics-based assays in insect and mammalian model systems.
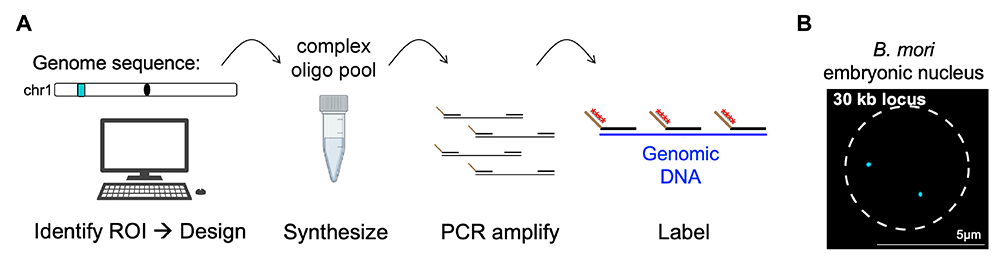
Figure 2. Oligopaint DNA FISH
A. Schematic of Oligopaint design workflow: Oligopaints are computationally designed chromosome paints that can be used for DNA or RNA fluorescence in situ hybridization (FISH). After designing probes using the Oligominer pipeline [Beliveau et al., Proc Natl Acad Sci USA 2012;109:21301; Beliveau et al., Proc Natl Acad Sci USA 2018;15, E2183–E2192] or PaintSHOP program [Hershberg et al., Nat Methods 2022;18:937], oligos are ordered as a complex oligo pool, amplified in the lab by PCR, and visualized using barcode-mediated secondary oligo labeling.
B. Example of Oligopaints in action. Shown is an embryonic nucleus (outlined by dashed line) labeled with a small 30 kb Oligopaint (cyan). The two dots indicate both maternal and paternal labeled chromosome copies.
Figure 2. Oligopaint DNA FISH
A. Schematic of Oligopaint design workflow: Oligopaints are computationally designed chromosome paints that can be used for DNA or RNA fluorescence in situ hybridization (FISH). After designing probes using the Oligominer pipeline [Beliveau et al., Proc Natl Acad Sci USA 2012;109:21301; Beliveau et al., Proc Natl Acad Sci USA 2018;15, E2183–E2192] or PaintSHOP program [Hershberg et al., Nat Methods 2022;18:937], oligos are ordered as a complex oligo pool, amplified in the lab by PCR, and visualized using barcode-mediated secondary oligo labeling.
B. Example of Oligopaints in action. Shown is an embryonic nucleus (outlined by dashed line) labeled with a small 30 kb Oligopaint (cyan). The two dots indicate both maternal and paternal labeled chromosome copies.
Transcriptional regulation in fly ovaries
Accurate transcriptional regulation in the germline is essential for proper development of gametes and future offspring. We are specifically interested in understanding the transcriptional programs involved in the differentiation of female germline stem cells into mature oocytes (Figure 3). This includes both the transcription of factors required for the development of the oocyte itself, but also the expression of RNAs and proteins that drive early embryonic development (maternally deposited RNAs and proteins). We have little insight into what activates the expression of these maternal factors. We are currently investigating several candidate transcription factors to determine their role in the female germline and early embryo using the model system Drosophila melanogaster.
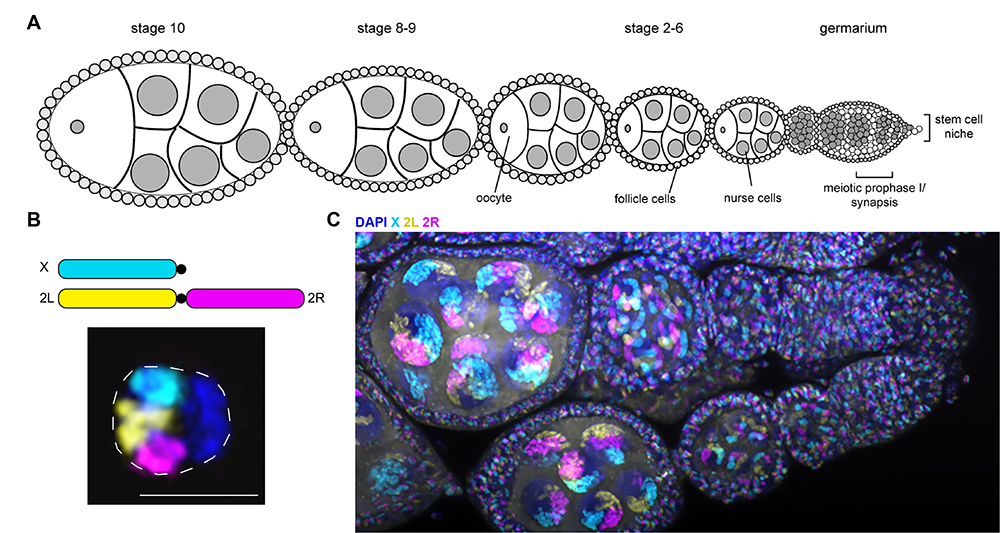
Figure 3. Drosophila oocyte development and fly chromosome painting
A. Schematic of one ovariole from a Drosophila ovary. Each ovary contains 15–20 ovarioles, which are linear arrays of developing egg chambers, with the tip (germarium; right) housing the mitotic germline stem cells and the oocytes and nurse cells during the early portion of the meiotic divisions. The most mature chambers are located most distal from the stem cell niche.
B. Top: Schematic of whole-chromosome Oligopaints labeling 3 of the 5 major fly chromosome arms. Bottom: Example of paints in fly cultured embryonic cell (Kc cell). Because homologous chromosome pairing is ubiquitous in Drosophila (even in somatic cells), the two chromosome copies are paired here, resulting in a single FISH per chromosome.
C. Example of the same Oligopaints shown in B, labeling all the cells in a fly ovary. Two ovarioles are shown in the image, with the germaria being on the right and more mature egg chambers toward the left. The large nuclei in each egg chamber are the polyploid nurse cells.
Figure 3. Drosophila oocyte development and fly chromosome painting
A. Schematic of one ovariole from a Drosophila ovary. Each ovary contains 15–20 ovarioles, which are linear arrays of developing egg chambers, with the tip (germarium; right) housing the mitotic germline stem cells and the oocytes and nurse cells during the early portion of the meiotic divisions. The most mature chambers are located most distal from the stem cell niche.
B. Top: Schematic of whole-chromosome Oligopaints labeling 3 of the 5 major fly chromosome arms. Bottom: Example of paints in fly cultured embryonic cell (Kc cell). Because homologous chromosome pairing is ubiquitous in Drosophila (even in somatic cells), the two chromosome copies are paired here, resulting in a single FISH per chromosome.
C. Example of the same Oligopaints shown in B, labeling all the cells in a fly ovary. Two ovarioles are shown in the image, with the germaria being on the right and more mature egg chambers toward the left. The large nuclei in each egg chamber are the polyploid nurse cells.
Regulation of meiotic recombination and crossover designation
The formation of crossovers (CO) during meiosis shuffles genes, but more importantly, it ensures that homologous chromosomes are accurately segregated. Inaccurate segregation can lead to aneuploidy in progeny or infertility. Crossover formation is tightly regulated and, in many species, COs are limited to one per chromosome pair (Figure 4). Interestingly, 10-fold more DNA double strand breaks (DSBs) form than COs in most species. How DSBs are designated to become COs is still unknown. We are utilizing two distantly related moths (silkworm moth and pantry moth) to study CO formation in meiosis. We are investigating differences in the CO regulation pathways between these moths using a combination of genomics, proteomics, and cytology. In a separate project, we are using cytology and genomics to uncover the causes of crossover suppression in Drosophila mutants. These studies will shed light on the overall process of CO formation and designation, with the goal of identifying therapeutic targets for humans with infertility related to defects in CO formation.
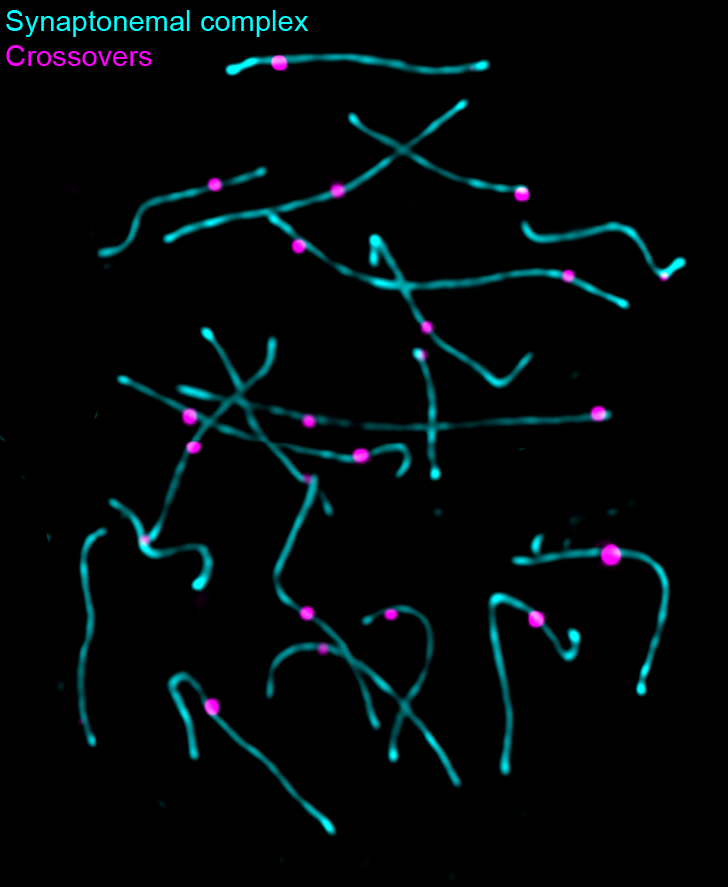
Figure 4. Crossovers in mouse spermatogenesis
Shown is a portion of a cell at the pachytene stage of meiotic prophase I from a mouse spermatocyte spread. This stage is where synapsis is complete and crossovers form. The synaptonemal complex is shown in cyan (anti-SYCP2) and the crossover sites are shown in magenta (anti-MLH1). Because of the tight regulation of crossovers, each chromosome contains only one (and rarely two) crossovers.
Figure 4. Crossovers in mouse spermatogenesis
Shown is a portion of a cell at the pachytene stage of meiotic prophase I from a mouse spermatocyte spread. This stage is where synapsis is complete and crossovers form. The synaptonemal complex is shown in cyan (anti-SYCP2) and the crossover sites are shown in magenta (anti-MLH1). Because of the tight regulation of crossovers, each chromosome contains only one (and rarely two) crossovers.
Mechanisms of meiotic chromosome segregation
Proper segregation of maternal and paternal chromosomes (homologs) during meiosis is critical for ensuring genome stability in the next generation and preventing reproductive complications such as infertility. However, our understanding of the mechanisms regulating homolog segregation in humans is incomplete, given that meiosis only occurs in a subset of specialized cells, making it difficult to study. Our lab is currently employing moth model systems, such as the pantry moth, to investigate meiosis-specific modes of chromosome segregation and kinetochore formation. Kinetochores are large protein complexes that facilitate chromosome segregation. Using cytological approaches, we found that moths form kinetochores along their entire chromosomes during mitosis (general cell division) but restrict kinetochores to chromosome centers during meiosis (Figure 5). We are working on elucidating how one system can employ several models of kinetochore assembly, findings that could also provide a basis for treatment for human infertility.
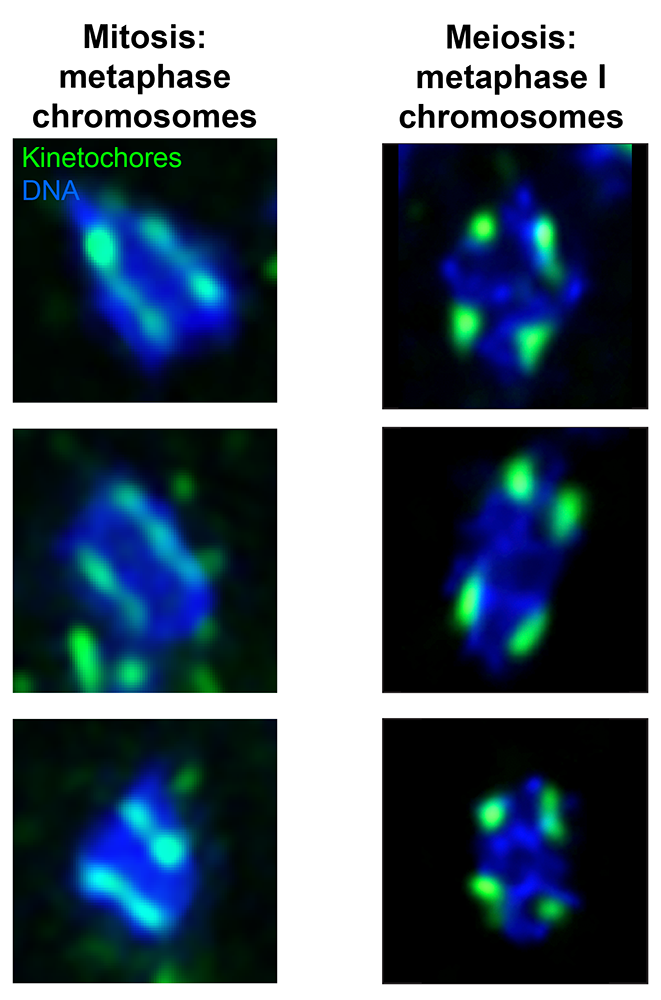
Figure 5. Kinetochore formation varies between mitosis and meiosis in moths.
Left: Three single metaphase chromosomes from mitotically dividing cultured cells from the moth P. interpunctella. Kinetochores (Dsn1) are shown in green, and DNA (DAPI) is shown in blue.
Right: Three single metaphase I (meiosis) chromosomes from P. interpunctella larval testis. Kinetochores are again shown in green, and DNA is shown in blue. In mitosis, kinetochores form all along the length of the chromosome (holocentric structure), while in meiosis, kinetochores are restricted to discrete loci on the chromosomal bivalent (two connect homologs). Images are from Reference 1.
Figure 5. Kinetochore formation varies between mitosis and meiosis in moths.
Left: Three single metaphase chromosomes from mitotically dividing cultured cells from the moth P. interpunctella. Kinetochores (Dsn1) are shown in green, and DNA (DAPI) is shown in blue.
Right: Three single metaphase I (meiosis) chromosomes from P. interpunctella larval testis. Kinetochores are again shown in green, and DNA is shown in blue. In mitosis, kinetochores form all along the length of the chromosome (holocentric structure), while in meiosis, kinetochores are restricted to discrete loci on the chromosomal bivalent (two connect homologs). Images are from Reference 1.
Generation of tools for studying germline biology
Tissue-specific CRISPR system
One of the major limitations of working in moth model systems is the lack of tools for genetic manipulation. While we can easily create whole-animal knockouts or knock-ins using CRISPR and piggyBac transposon technologies, respectively, targeting meiotic genes that may also be involved in somatic processes often leaves whole-animal mutants inviable. To overcome this obstacle, we are generating a tissue-specific CRISPR system in which CRISPR/Cas9 is expressed using a germline-specific promoter. Thus, even when injections are done in single-cell embryos, the mutations will only impact germline cells. However, given the incomplete state of the pantry moth genome annotations, it is not entirely clear which commonly used Drosophila germline promoters are conserved. Therefore, we are performing single-cell “multiomics” in pantry moth ovaries and testes, which combines 10x ATAC-Seq and 10x RNA-Seq to obtain transcription and accessibility information at single-cell resolution. By comparing these germline data sets with our somatic cell data sets, we can identify genes and promoters that are expressed specifically in the germline. We will target these genes using a candidate-based CRISPR knockout screening and assess associated fertility defects. Genes identified as being essential for fertility will be further dissected using cytological and additional genomics assays. The project will not only generate new tools but may also identify novel meiotic genes for future studies.
Identification of phenotypic markers for co-CRISPR
CRISPR co-targeting (co-CRISPR) has simplified the process of crossing and genotyping G1 offspring. The technique hinges on the co-occurrence of editing at a phenotypic marker and a gene-of-interest (GOI). In the pantry moth, loss-of-function mutations in pigment genes are typically used for co-CRISPR experiments (Figure 6). However, some of these mutations result in a loss of pigment from the testes, making the meiotically active larval stage difficult to sex. To circumvent this, we are testing alternative phenotypic markers (such as ebony and Tubby) to be used in co-CRISPR knockout experiments to indicate co-editing of the genes involved in meiosis and germ cell development. These tools will allow for enrichment of G0 founders likely to carry germline mutation at a GOI, allowing us to easily assess lethality and/or fertility, and further characterize a wide variety of meiotic-specific and developmental genes.
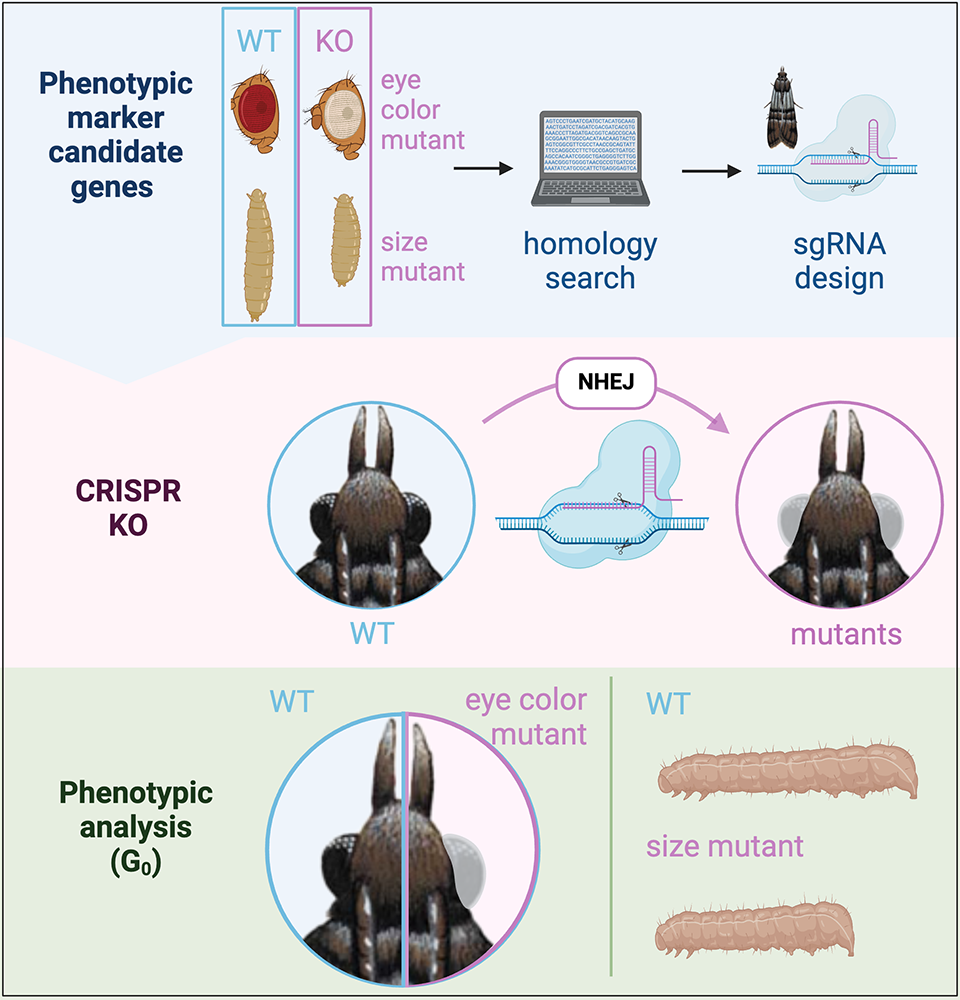
Figure 6. Knockout of phenotypic markers for co-CRISPR experiments
To find new phenotypic markers for co-CRISPR experiments, we are identifying conserved genes that are used in phenotypic markers in flies (for example, new color mutants or the Tubby body size mutant) in the P. interpunctella genome (top). We then design guide RNAs to these genes to create knockouts (top and middle), and we can phenotype larvae or adults (bottom) to determine the success of our CRISPR experiments. Figure adapted from Heryanto C et al., Front Genome Ed 2022;4:1074888; Heryanto C et al., iScience 2022;25:103885.
Figure 6. Knockout of phenotypic markers for co-CRISPR experiments
To find new phenotypic markers for co-CRISPR experiments, we are identifying conserved genes that are used in phenotypic markers in flies (for example, new color mutants or the Tubby body size mutant) in the P. interpunctella genome (top). We then design guide RNAs to these genes to create knockouts (top and middle), and we can phenotype larvae or adults (bottom) to determine the success of our CRISPR experiments. Figure adapted from Heryanto C et al., Front Genome Ed 2022;4:1074888; Heryanto C et al., iScience 2022;25:103885.
Publications
- Chromosome segregation during spermatogenesis occurs through a unique center-kinetic mechanism in holocentric moth species. PLOS Genet 2024 20(6):e1011329
- Review: uncharted territories: solving the mysteries of male meiosis in flies. PLOS Genet 2024 20(3):e1011185
- An egg-sabotaging mechanism drives non-Mendelian transmission in mice. Curr Biol 2024 34(17):3845–3854
Collaborators
- Takashi Akera, PhD, Laboratory of Chromosome Dynamics and Evolution, NHLBI, Bethesda, MD
- Elissa Lei, PhD, Nuclear Organization and Gene Expression Section, NIDDK, Bethesda, MD
Contact
For more information, email leah.rosin@nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/rosin.