Molecular Mechanisms of Synapse Assembly and Homeostasis
- Mihaela Serpe,
PhD, Head, Section on Cellular Communication - Peter Nguyen, Biological Laboratory Technician
- Tae Hee Han, PhD, Staff Scientist
- Rosario Vicidomini, PhD, Research Fellow
- Shefali Goyal, PhD, Visiting Fellow
- Wen Chieh Hsieh, PhD, Visiting Fellow
- Debabrata Sinha, PhD, Visiting Fellow
- Jenny Huang, BA, Postbaccalaureate Fellow

The purpose of our research is to understand the mechanisms of synapse development and homeostasis. The chemical synapse is the fundamental nervous-system communication unit that connects neurons to one another and to non-neuronal cells and is designed to mediate rapid and efficient transmission of signals across the synaptic cleft. Crucial to this function is the ability of a synapse to change its properties, so that it can optimize its activity and adapt to the status of the cells engaged in communication and/or to the larger network comprising them. Consequently, synapse development is a highly orchestrated process coordinated by intercellular communication between the pre- and postsynaptic compartments and by neuronal activity itself. Our long-term goal is to elucidate the molecular mechanisms that regulate assembly of functional synapses during development and their fine-tuning during maturation, plasticity, and homeostasis. We use a comprehensive set of approaches that include genetics, biochemistry, molecular biology, cell-biology, super-resolution imaging, and electrophysiology recordings in live animals and in reconstituted systems. In recent studies, we also utilized single-cell RNA sequencing (scRNA-seq) methodologies to describe various populations of neurons.
Because of its many advantages, we use the Drosophila neuromuscular junction (NMJ) as a model for glutamatergic synapse development and function. The fact that individual NMJs can be reproducibly identified from animal to animal and are easily accessible for electrophysiological and optical analysis makes them uniquely suited for in vivo studies on synapse assembly, growth, and plasticity. In addition, the richness of genetic manipulations that can be performed in Drosophila permits independent control of individual synaptic components in distinct cellular compartments. Importantly, the fly NMJ relies entirely on kainate-type receptors, a family of ionotropic glutamate receptors that impact synaptic transmission and neuronal excitability in the mammalian central nervous system but remain poorly understood. The Drosophila NMJ can thus be used to analyze and model defects in the structural and physiological plasticity of glutamatergic synapses, which are associated with a variety of human pathologies, from learning and memory deficits to autism. Drosophila has long served as a source of insight into human genetics, development, and disease. The basic discoveries of our laboratory makes in the fly will serve our overarching goal of understanding how chemical synapses are assembled and sculpted during development and homeostasis.
A single-cell RNA-seq atlas of the Drosophila larval ventral cord
The Drosophila larval ventral nerve cord (VNC) has many similarities with the spinal cord of vertebrates and has emerged as a major model for understanding the development and function of motor systems. In flies, as in vertebrates, neuronal activity induces input-specific changes in the synaptic strength; at the larval NMJ, the postsynaptic sensitivity is primarily modulated via synapse-specific recruitment of postsynaptic glutamate receptors. Robust homeostatic mechanisms keep synapses within an appropriate dynamic range such that the evoked potentials measured in the muscle remain constant from embryo to third instar larval stages.
To learn how motor neurons (MNs) respond to different signaling inputs or compensate for various postsynaptic perturbations, we wish to compare the scRNA-seq profile of MNs in control and mutant animals, in which various signaling pathways have been perturbed. To comprehensively analyze the effects of perturbations and to create a community resource for broader studies on the VNC, we elected to carry out scRNA-seq on all cells of the larval VNC. The work was conducted with support from the NICHD Genomics Core and in collaboration with Steve Coon, Fabio Rueda Faucz, and James Iben.
To simplify cell type identification, we marked different neuron populations with reporters (i.e., vGlut-RFP) or the Gal4 system, processed five different genotypes (w1118, twit-Gal4→UAS-GFP, vGlut-RFP, RN2-Gal4→UAS-GFP, and Proc-Gal4→UAS-GFP), and then combined them in a dataset that captures biological trends common to all genetic backgrounds. The initial clusters were manually curated and aggregated into 18 distinct cell populations including several glia subtypes, interneurons, MNs, and a population of immature cells from neural progenitors to their progeny that will become adult neurons (Figure 1).
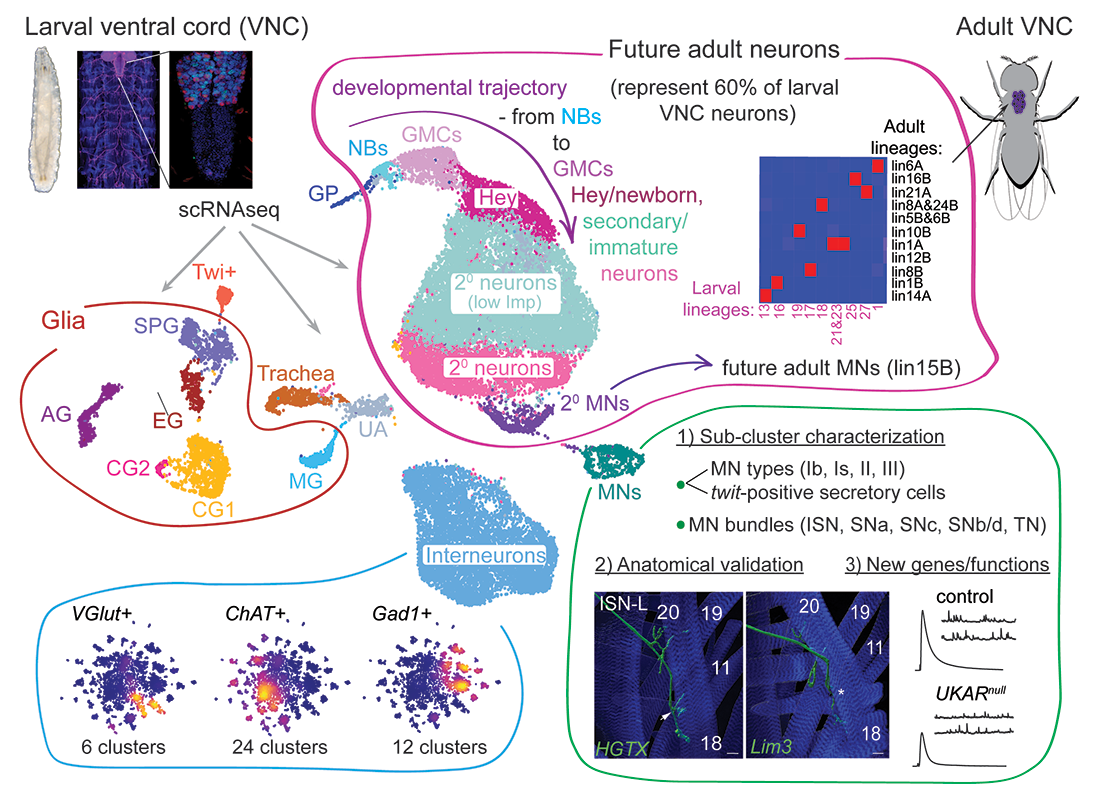
Figure 1. Single cell RNA-seq atlas of Drosophila larval VNC – a graphical abstract
Two-dimensional representation (UMAP) of 31,040 VNC cells from five different samples. Several key comparisons and functional analyses performed are indicated and superimposed onto the UMAP plot. Each dot represents a single cell. Each cell type was assigned based on the expression of known markers and illustrated by a unique color. GPs: glia precursors; NBs: neuroblasts; GMCs: ganglion mother cells; MNs: motor neurons; MD: midline glia; AG: astrocyte-like glia; EG: ensheathing glia; SPG: subperineurial glia; CG1 and CG2: cortex glia 1 and 2.
Figure 1. Single cell RNA-seq atlas of Drosophila larval VNC – a graphical abstract
Two-dimensional representation (UMAP) of 31,040 VNC cells from five different samples. Several key comparisons and functional analyses performed are indicated and superimposed onto the UMAP plot. Each dot represents a single cell. Each cell type was assigned based on the expression of known markers and illustrated by a unique color. GPs: glia precursors; NBs: neuroblasts; GMCs: ganglion mother cells; MNs: motor neurons; MD: midline glia; AG: astrocyte-like glia; EG: ensheathing glia; SPG: subperineurial glia; CG1 and CG2: cortex glia 1 and 2.
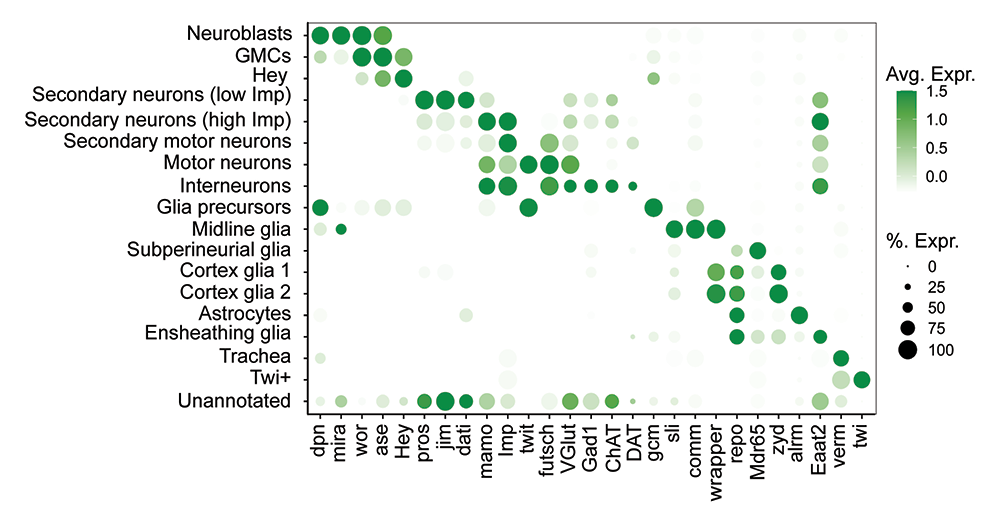
We found that only 40% of the larval VNC cells (13,120 out of a total of 31,040 single-cell transcriptomes) are mature and could fulfill larval functions. The other 60% (17,920) are dedicated to establishing the VNC populations needed during adult stages. These immature cells include neural progenitors, neuroblasts (NBs), and their progenies that will become adult neurons. Using cell-type–defining markers (Figure 2), we captured a developmental trajectory that included NBs (marked by the presence of dpn, mira, wor, and ase), their immediate progeny ganglion mother cells (GMCs) (wor, ase), newborn neurons (Hey), and immature secondary neurons at two distinct developmental stages, first with lower levels of Imp (jim, dati, pros, and “low Imp”) and second with high levels of Imp expression (mamo, “high Imp”).
Figure 2. Markers for identification of larval VNC cell types
Dot plot of marker genes for each major cell type. The dot size represents the percent of cells that express each gene of interest (GOI) within the cluster. The color intensity reflects the average expression level.
Figure 2. Markers for identification of larval VNC cell types
Dot plot of marker genes for each major cell type. The dot size represents the percent of cells that express each gene of interest (GOI) within the cluster. The color intensity reflects the average expression level.
Two clusters of MNs (marked by futsch, VGlut, lbm, Proc, and rhea) include primary larval MNs, that are metabolically active and express components of active synapses, and secondary MNs, which are metabolically and functionally silent. Upon synaptogenesis, primary MNs receive muscle-derived BMP (bone morphogenetic protein) signals, which coordinate synapse development through transcriptional modulation. Indeed, primary MNs express BMP–target genes such as twit; by contrast, secondary MNs show no twit expression, indicating that they lack retrograde BMP signaling at this stage.
A large cluster of putative interneurons contains heterogeneous, active neurons expressing distinct neurotransmitter markers (VGlut, ChAT, Gad1, and DAT). We identified several glia subtypes, including glia precursors (GPs) (gcm), midline glia (sli, comm, and wrapper), subperineurial glia (Mdr65), cortex glia (wrapper, zyd), astrocytes (alrm), and ensheathing glia (Eaat2). Our atlas contains 3,148 glia, which represented 10.1% of the VNC cells recovered. We also identified a cluster of trachea cells and a cluster that exclusively expresses the mesodermal marker twi (Twi+ cluster). Despite stringent quality controls, we detected a non-uniform population of cells (UA, unannotated; cluster 15), but which was excluded from further analyses.
This atlas captures the cellular diversity of larval VNC, with each cell type characterized by a high number of transcripts. It also reveals distinct cellular functions and metabolic features. For example, MNs have the most complex transcriptomes: their high-energy needs correlate with enhanced expression of glycolysis genes. The secondary neurons are small and have fewer ribosomes. Thus, our atlas can distinguish among different cell types, cell states, and functional characteristics within the larval VNC.
Primary and secondary MNs
Larval MNs have been studied for almost 50 years, as their accessibility and stereotyped morphology facilitate in-depth analyses. However, to date there is no systematic transcriptome analysis or class-specific characterization of larval MNs. Each abdominal hemisegment contains 30 body-wall muscles innervated by MNs of four classes: type Ib and Is glutamatergic MNs, type II octopaminergic neurons, and type III peptidergic neurons. Each muscle is innervated by a dedicated Ib MN, the primary driver for contraction. Type Is MNs, marked by the cell-adhesion molecule DIP-ɑ, innervate groups of 7–8 muscles and coordinate contractions. Type II modulatory MNs secrete octopamine and express enzymes involved in octopamine biosynthesis and transport (Tdc2, Tbh, and Vmat). Type III peptidergic neurons secrete neurohormones (Burs, CCAP). Given that BMP signaling is also required for peptidergic cell-fate specification, twit expression could be used to capture all these neuronal types (Figure 3A-B).
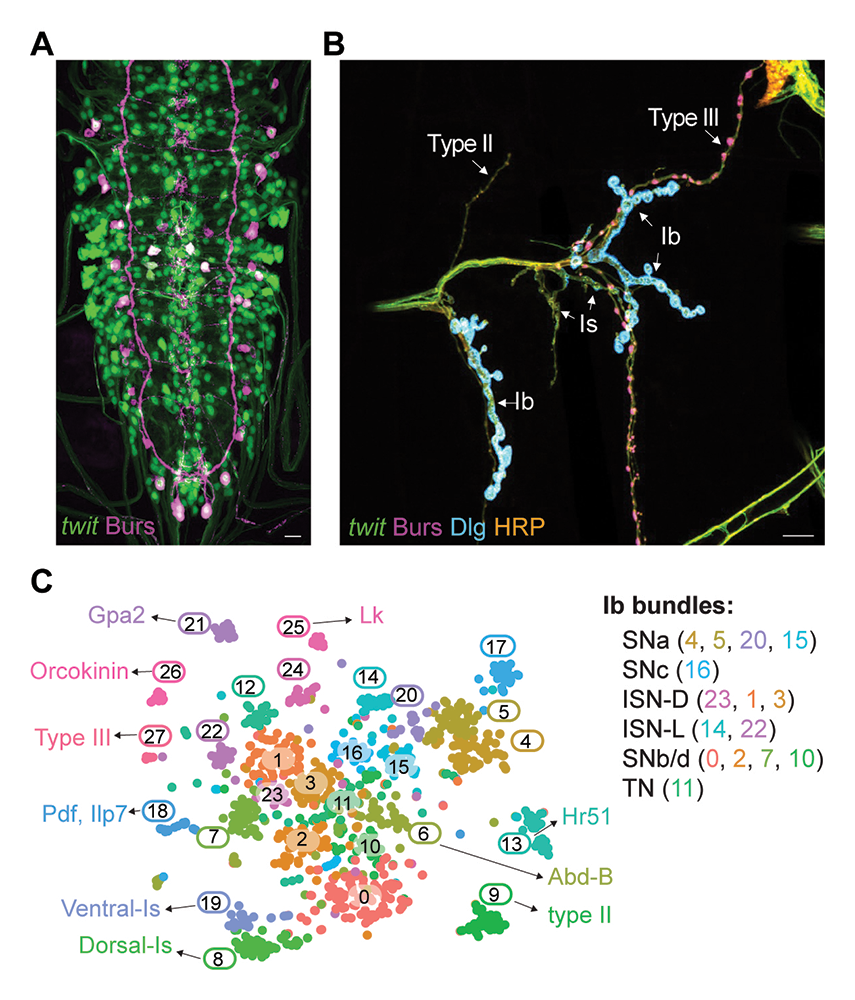
Figure 3. Labeling and identification of MN subtypes
A-B. Confocal images of third instar larva VNC and NMJ stained for twit (green), Burs (magenta), Dlg (cyan), and HRP (orange). Different types of MNs are indicated: type Ib, large boutons surrounded by rich Dlg staining; type Is, small boutons with reduced Dlg staining; type II, tiny boutons; and type III, Burs–positive oval boutons.
C. tSNE (t-distributed stochastic neighbor embedding) plot of all twit clusters, indicating assigned and proposed identities for all 28 clusters; twit (target of wit) encodes a GPI–anchored membrane ligand of the Ly6 family.
Figure 3. Labeling and identification of MN subtypes
A-B. Confocal images of third instar larva VNC and NMJ stained for twit (green), Burs (magenta), Dlg (cyan), and HRP (orange). Different types of MNs are indicated: type Ib, large boutons surrounded by rich Dlg staining; type Is, small boutons with reduced Dlg staining; type II, tiny boutons; and type III, Burs–positive oval boutons.
C. tSNE (t-distributed stochastic neighbor embedding) plot of all twit clusters, indicating assigned and proposed identities for all 28 clusters; twit (target of wit) encodes a GPI–anchored membrane ligand of the Ly6 family.
We labeled primary MNs via twit→GFP and performed scRNA-seq on FACS–sorted cells. The integrated MN population was subdivided into 28 clusters including well defined type II MNs (cluster 9: Vmat, Tdc2, and Tbh), type III (cluster 27: Burs, CCAP), and additional neuroendocrine cells (cluster 25: Leucokinin [LK], cluster 21: Gpa2, and cluster 26: Orcokinin [OK]). This clear separation permitted the identification of many genes that may shape the function of these cells. For example, both type II and type III neurons receive glutamate input via AMPA receptors (GluRIA, GluRIB). Type III neurons secrete hormones required for developmental timing, but they also emerge as key regulators of sleep onset and homeostasis. The neuroendocrine cells (clusters 21, 25, and 26) express additional neuropeptides that may act as neurotransmitters or neuromodulators. In all bilateria, Hox genes specify structures along the antero-posterior axis. Serial homologs of each neuron exist in each segment and express different Hox genes, but unsupervised clustering of limited numbers of cells favors functional homology over spatial patterning. However, when we examined specific clusters, for example the type II neurons from all abdominal segments, the heterogeneous expression of Hox genes was clearly captured, consistent with different Hox genes expressed along the anterior-posterior axis.
Notably, two related MN clusters, cluster 8 and cluster 19, express the Is marker DIP-ɑ; in conjunction with sets of transcription factors (TFs) associated with ventrally or dorsally projecting MNs, we conclude that cluster 8 contains dorsal Is (D-Is) and cluster 19 ventral Is (V-Is) MNs. Further inspection of differentially expressed genes combined with anatomical examination permitted the identification of all twit clusters. A summary of validated and proposed identities is shown in Figure 3C. A closer comparison of type Ib and Is MNs revealed that, while they express type-specific TFs and RNA–binding proteins, most transcripts are present in both MN types and are elevated in one group or another, suggesting that these highly related MNs have differentially tuned functions. The main differences include Ca2+ sensing and buffering mechanisms and cell-adhesion molecules.
The Drosophila larval NMJ has been an excellent model for genetic and morphological analyses of synapse development and homeostasis. However, the features of various MN subtypes have been largely inaccessible using classic scRNA-seq methodologies because larval VNC contains relatively few primary MNs. Through reiterative sets of FACS sorting, sequencing, and validation, we increased the number of primary MNs, resolved them into individual bundles/neurons, and linked them to morphologically characterized known subtypes. This excellent resolution revealed functional variations among MNs, provided information on signaling and connectivity at the cellular and molecular level, captured the diversity of neurotransmitter receptors, and uncovered new candidates for cell specification, development, and function.
Our atlas provides a high-resolution characterization of larval VNC, capturing primary neurons, glia, and the functional landscape that coordinates larval behavior. At the same time, the atlas offers unique insights into neurogenesis and into the strategies and signaling networks utilized for the generation of the adult VNC. As illustrated by numerous examples, our atlas represents a resource for data mining and hypothesis generation, a powerful platform for future discoveries of cellular and molecular mechanisms in repair, regeneration, plasticity, homeostasis, and behavioral coordination.
The gating properties of Drosophila NMJ glutamate receptors and their dependence on Neto
Historically, a major obstacle to understanding ionotropic glutamate receptor (iGluR) function was the inability to reconstitute functional Drosophila NMJ receptors in heterologous systems and dissect their properties. Given that iGluRs recruitment and synaptic stabilization depend on their gating properties, such functional analyses are critical for comprehending the compound in vivo phenotypes of mutants with perturbed synaptic activities. We previously discovered that an obligatory auxiliary protein, Neto, is absolutely required for clustering of postsynaptic iGluRs and NMJ functionality. Neto belongs to a family of highly conserved auxiliary proteins that share an ancestral role in the formation and modulation of glutamatergic synapses. Vertebrate Neto1 and Neto2 were shown to modulate the properties of selective iGluRs, in particular kainate-type receptors. The fly NMJ relies exclusively on kainate-type receptors, making this genetically powerful system completely dependent on Neto activities.
Drosophila neto codes for two isoforms, Neto-α and Neto-β, which share the extracellular and transmembrane domains but have distinct intracellular parts. Our discovery of Drosophila Neto was a key step towards the functional reconstitution of recombinant NMJ receptors. Thus, in previous work we found that, when expressed in Xenopus oocytes, NMJ iGluRs in complex with Neto form rapidly desensitizing Ca2+–permeable channels with low affinity for glutamate. In the absence of Neto, the NMJ iGluRs reach the cell surfaces but elicit no glutamate-gated currents. More recently, we expressed functional NMJ iGluR/Neto complexes in HEK cells (an immortalized cell line), in which the biophysical properties of these receptors can be further dissected using outside-out patches and fast agonist perfusion. As in Xenopus oocytes, Neto is critical for the functional reconstitution of both postsynaptic receptors (type-A: GluRIIA/C/D/E and type-B: GluRIIB/C/D/E): 100% of the patches (n>100) yielded no currents in the absence of Neto proteins.
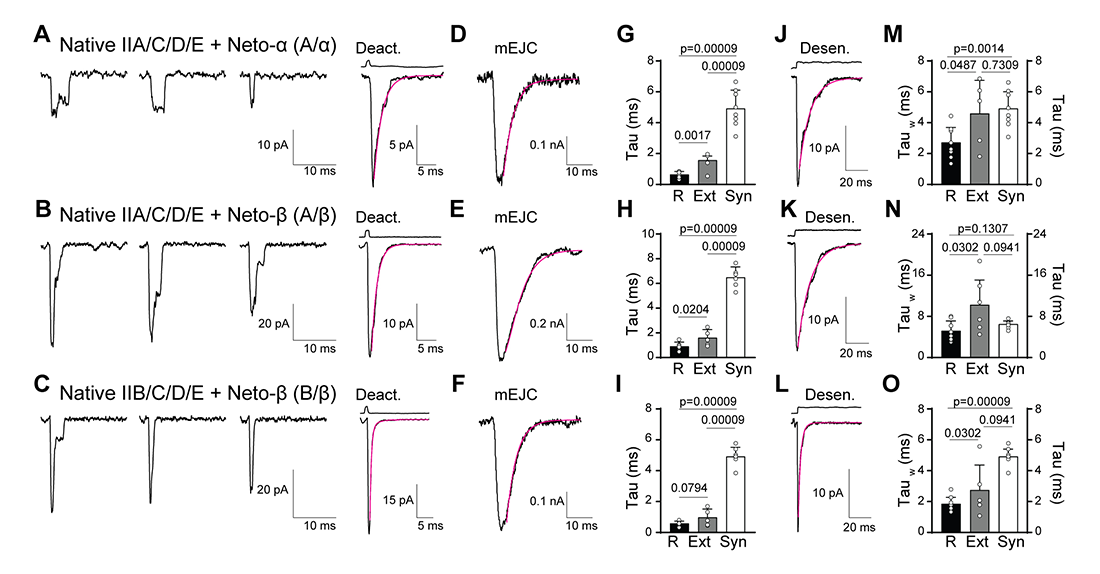
We next compared the properties of recombinant receptors (expressed in HEK cells) with native extra-junctional and synaptic receptors of genetically controlled composition (Figure 4). We found that the type-A and type-B receptors have comparable deactivation rates, but type-B desensitize four times faster than do type-A. Neto isoforms differentially modulate these channels, adding further diversity to their functional repertoire. For example, Neto-α accelerates the desensitization of both channels whereas Neto-β and Neto-ΔCTD confer similar kinetics. This confirms our earlier proposal that Neto-β functions primarily as an organizing scaffold at the NMJ. Surprisingly, we found that deactivation is extremely fast and that the decay of synaptic currents resembles the rate of iGluR desensitization. This indicates that: (1) the clearance of glutamate from the synaptic cleft is slow; (2) additional auxiliary or modulatory proteins are present and impact the gating of synaptic receptors; and (3) desensitization plays a major role in determining the kinetics of synaptic transmission at the larval NMJ.
Figure 4. Distinct receptor properties observed for native and recombinant receptors
A-C. Representative individual traces (left) and the average of 20–40 responses (right) to 10 mM glutamate applied for 1 ms to outside-out patches of native, extra-junctional receptors excised from body-wall muscles of third instar larvae with distinct iGluR/Neto complexes, as indicated. The magenta line shows time course of deactivation fit by an exponential function; open tip junction currents measured at the end of the experiments are shown at the top.
D-F. Miniature EJCs (excitatory junction currents) recorded from larvae of the same genotypes fit with single exponential functions.
G-I. Comparisons of deactivation kinetics for recombinant receptors expressed in HEK cells (R), native, extra-junctional receptors from larval muscle membranes (Ext) and mEJCs (Syn).
J-L. Average of 20–40 responses to 10 mM glutamate applied for 100 ms to outside-out patches of native, extra-junctional receptors for the same genotypes; the magenta line shows fits of the sum of two exponential functions; open tip junction currents measured at the end of the experiments are shown at the top.
M-O. Comparisons of desensitization kinetics for recombinant receptors expressed in HEK cells (R), native, extra-junctional receptors from larval muscle membranes (Ext) and mEJCs (Syn). Data are represented as mean ± SD.
Figure 4. Distinct receptor properties observed for native and recombinant receptors
A-C. Representative individual traces (left) and the average of 20–40 responses (right) to 10 mM glutamate applied for 1 ms to outside-out patches of native, extra-junctional receptors excised from body-wall muscles of third instar larvae with distinct iGluR/Neto complexes, as indicated. The magenta line shows time course of deactivation fit by an exponential function; open tip junction currents measured at the end of the experiments are shown at the top.
D-F. Miniature EJCs (excitatory junction currents) recorded from larvae of the same genotypes fit with single exponential functions.
G-I. Comparisons of deactivation kinetics for recombinant receptors expressed in HEK cells (R), native, extra-junctional receptors from larval muscle membranes (Ext) and mEJCs (Syn).
J-L. Average of 20–40 responses to 10 mM glutamate applied for 100 ms to outside-out patches of native, extra-junctional receptors for the same genotypes; the magenta line shows fits of the sum of two exponential functions; open tip junction currents measured at the end of the experiments are shown at the top.
M-O. Comparisons of desensitization kinetics for recombinant receptors expressed in HEK cells (R), native, extra-junctional receptors from larval muscle membranes (Ext) and mEJCs (Syn). Data are represented as mean ± SD.
Similar to Ca2+–permeable vertebrate kainate receptors, application of Philanthotoxin (PhTx), a toxin widely used for acute homeostasis studies at larval NMJ, caused a substantial block for all iGluR/Neto complexes. Application of the lectin Concanavalin A attenuates desensitization and increases the open time for all receptors; this facilitated isolation of single-channel currents with well defined sub-conductance states, a widely observed feature of the gating of vertebrate AMPA and kainate receptors. Understanding single-channel behavior is key to revealing how synapses function and how auxiliary proteins control receptor gating and conductance properties.
The fly NMJ is a powerful genetic model for investigating conserved mechanisms for synapse assembly, development, and homeostasis. Unlike in vertebrates, where in vivo studies of kainate receptors have been technically highly challenging, disruption of kainate receptor biology at the fly NMJ triggers a wide range of morphological and behavioral phenotypes, from a complete loss of postsynaptic receptors and synaptic structures, which causes embryonic paralysis and developmental lethality, to defects in the assembly and maintenance of postsynaptic densities, which generally induce locomotor deficits, and finally to subtle changes in postsynaptic composition and impairments in synaptic plasticity. Within this wide range of biological outcomes, investigations of fly NMJ iGluRs and their modulation by Neto have the potential to reveal new functions for iGluRs and Neto and new modalities of regulation.
Publications
- scRNA-seq data from the larval Drosophila ventral cord provides a resource for studying motor systems function and development. Dev Cell 2024 59(9):1210–1230
- Versatile nanobody-based approach to image, track and reconstitute functional Neurexin-1 in vivo. Nat Commun 2024 15:6068
- The gating properties of Drosophila NMJ glutamate receptors and their dependence on Neto. J Physiol 2024 602(24):7043–7064
Collaborators
- Steven Coon, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- James Iben, PhD, Molecular Genetics Core, NICHD, Bethesda, MD
- Dragan Maric, PhD, Flow and Imaging Cytometry Core, NINDS, Bethesda, MD
- Mark L. Mayer, PhD, Section on Neurophysiology and Biophysics, NINDS, Bethesda, MD
- Felipe Opazo, PhD, Center for Biostructural Imaging of Neurodegeneration, Göttingen, Germany
- Fabio Rueda Faucz, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
Contact
For more information, email mihaela.serpe@nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/Serpe.