Cellular and Molecular Mechanisms of Lymphatic Disorders
- Sarah E. Sheppard,
MD, PhD, MST, Head, Unit on Vascular Malformations - Andrea Bowling, NP, Nurse Practitioner
- Deena Zeltser, MD, Staff Clinician
- Christopher Marshall, BA, Research Specialist II
- Luciana Daniela Garlisi Torales, MD, Postdoctoral Fellow
- Lola Zerbib, PharmD, PhD, Postdoctoral Fellow
- Jessica Johnson, BA, Predoctoral Fellow (Ox-Cam)
- Kwabena Aboagye, BS, Postbaccalaureate Fellow
- Georgia Krikorian, BA, Postbaccalaureate Fellow
- Ben Sempowski, BS, Postbaccalaureate Fellow
- Rachel Amedume, Howard University Undergraduate Honors Program Biology Student

The primary goal of our translational research group is to develop efficacious therapies for patients with complex lymphatic anomalies. To do this, we seek to understand the molecular etiologies of these complex lymphatic malformations, how the molecular etiologies alter molecular signaling, and how this affects the cellular mechanisms regulating normal lymphatic development. Ultimately, these answers will allow us to develop novel therapies.
Complex lymphatic anomaly is a term that encompasses four different complex lymphatic malformations: central conducting lymphatic anomaly (CCLA); generalized lymphatic anomaly (GLA); Kaposiform lymphangiomatosis (KLA); and Gorham Stout disease (GSD). Patients suffer from symptoms such as pleural effusions, pericardial effusions, ascites, and bone lesions, which can cause significant morbidity and even death. Currently, there is only one medication approved for patients with complex lymphatic anomalies caused by PIK3CA, a gene mutation known known to cause lymphatic malformations. Similar, precision-medicine approaches are needed for patients with other complex lymphatic anomalies.
Research in our lab will combine patient studies and genomics with the zebrafish model to identify novel therapies. The zebrafish model allows us to manipulate the genetics so as to rapidly create patient-based models, image the developing vasculature, understand cellular dynamics in vivo, and perform drug screening.
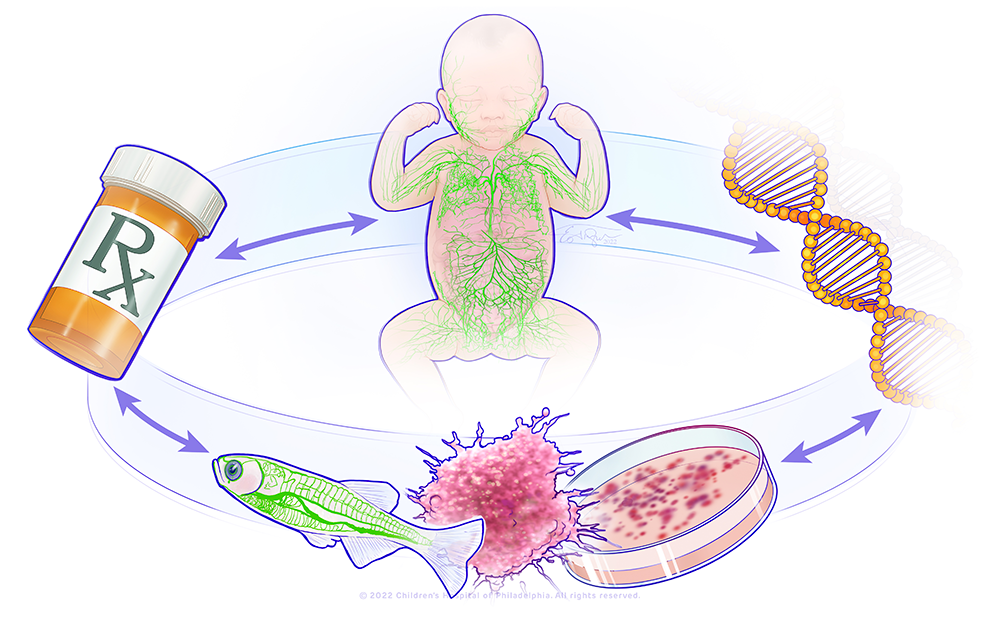
Figure 1. A bedside-to-bench precision medicine program for lymphatic anomalies
The figure shows a circle with the major components of the research in the lab: a child with abnormal lymphatics, a DNA molecule, a representation of a zebrafish model and organoid model, and a pill bottle. This represents a bedside-to-bench-to-bedside program using organoid and zebrafish to model patients' lymphatic anomalies and develop therapies that will be translated back to the patients.
Figure 1. A bedside-to-bench precision medicine program for lymphatic anomalies
The figure shows a circle with the major components of the research in the lab: a child with abnormal lymphatics, a DNA molecule, a representation of a zebrafish model and organoid model, and a pill bottle. This represents a bedside-to-bench-to-bedside program using organoid and zebrafish to model patients' lymphatic anomalies and develop therapies that will be translated back to the patients.
Natural history study of lymphatic disorders
In 2023, the lab launched a prospective natural history study for individuals with lymphatic anomalies to systematically evaluate the disease phenotypes and long-term outcomes. As these are rare diseases, patients may often face misdiagnosis. Moreover, there is a lack of information about the long-term outcomes. Patients are seen by telemedicine by the primary team or at the NIH Clinical Center by the primary team and several specialists for deep phenotyping. The study will allow us to provide improved prognostication to families, establish screening/monitoring guidelines, determine best practices for genetic diagnosis, and explore fertility outcomes for those on long-term medication management. The study will allow us to identify novel end-points for future clinical trials.
Genotype-phenotype correlations in lymphatic malformations
Turner syndrome (45,X) is caused by a complete or partial absence of a single X chromosome. Vascular malformations occur as a result of abnormal development of blood and/or lymphatic vessels. They arise from either somatic or germline pathogenic variants in the genes regulating the growth and apoptosis of vascular channels. Aortic abnormalities are a common, known vascular anomaly of Turner syndrome. However, previous studies have described other vascular malformations as a rare feature of Turner syndrome and suggested that vascular abnormalities in individuals with Turner syndrome may be more generalized. In this study, we described two individuals with co-occurrence of Turner syndrome and vascular malformations with a lymphatic component. In these individuals, genetic testing of the lesional tissue revealed a somatic pathogenic variant in PIK3CA, a known and common cause of lymphatic malformations. Based on this finding, we concluded that the vascular malformations presented here and likely those previously reported in the literature are not a rare part of the clinical spectrum of Turner syndrome, but rather a separate clinical entity that may or may not co-occur in individuals with Turner syndrome.
Therapies for KRAS–related lymphatic disorders
Previously, we identified that activating variants in the KRAS gene cause complex lymphatic anomalies. KRAS is a RAS-GTPase, and pathogenic variants are known to drive cancers as well as developmental disorders. Previously, we showed that KRAS variants can drive lymphatic malformations in the zebrafish, which can be treated with MEK (kinase that phosphorylates mitogen-activated protein kinase) inhibitors.
However, issues regarding therapies for KRAS–related lymphatic anomalies still remain. First, different variants in KRAS lead to up-regulation of MAPK signaling (a central signaling pathway) by different mechanisms. Second, the patients have different phenotypes. Third, previous patients with KRAS–related lymphatic anomalies have mixed responses to MEK inhibitors. Therefore, we developed new zebrafish models for three different KRAS–related variants. Currently, the lymphatic phenotypes are being delineated, and these models will be used for drug-therapy screening.
Cellular and molecular mechanisms of lymphatic malformations
Novel candidate genes and known genetic causes associated with disease must be validated to ensure that they truly cause disease. Mechanisms of disease must be elucidated for known causes of lymphatic disease as a first step toward identifying a precision-based treatment. Previously, we identified pathogenic variants in the RIT1 gene (RIT1 belongs to the Ras family of GTPases) as a novel cause of CCLA (Liu et al. Eur J Hum Genet 2022;30:1022). However, the mechanisms that drive disease are unknown. Therefore, we created a mosaic zebrafish model of RIT1 lymphatic anomalies and a zebrafish knock-in with a patient allele to evaluate the lymphatic anomalies, so as to determine the effect on the cellular and molecular mechanisms driving lymphatic development, and, in the future, to identify therapies.
Additional Funding
- NIH Distinguished Scholars Program (FY24)
- NICHD DIR Developing Talent Scholar (Georgia Krikorian)
Publications
- Lymphatic disorders caused by mosaic, activating KRAS variants respond to MEK inhibition. JCI Insight 2023 8(9):e155888
- Microcystic lymphatic malformations in Turner syndrome are due to somatic mosaicism of PIK3CA. Am J Med Genet A 2024 194(1):64–69
- Central conducting lymphatic anomaly: from bench to bedside. J Clin Invest 2024 134(8):e172839
- The importance of patient-specific resources for families dealing with prenatal rare diseases. Am J Med Genet A 2024 194(3):e63450
Contact
For more information, email sarah.sheppard@nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/sheppard.