Thyroid Hormone Regulation of Vertebrate Postembryonic Development
- Yun-Bo Shi,
PhD, Head, Section on Molecular Morphogenesis - Liezhen Fu, PhD, Staff Scientist
- Nga Luu, MS, Biologist
- Lingyu Bao, PhD, Visiting Fellow
- Suresh Babu Munipalli, PhD, Visiting Fellow
- Paisley Thomson, PhD, Postdoctoral Fellow
- Yuta Tanizaki, PhD, Visiting Fellow
- Emeric Louis, MS, Graduate Student
- Zhaoyi Peng, BS, Graduate Student

This laboratory investigates the molecular mechanisms of thyroid hormone (TH) function during postembryonic development, a period around birth in mammals when plasma TH levels peak. The main model is the metamorphosis of pseudo-tetraploid Xenopus laevis and diploid Xenopus tropicalis, two highly related species that offer unique but complementary advantages. The control of this developmental process by TH offers a paradigm to study gene function in postembryonic organ development. During metamorphosis, different organs undergo vastly different changes. Some, such as the tail, undergo complete resorption, while others, such as the limb, are developed de novo. The majority of the larval organs persist through metamorphosis but are dramatically remodeled to function in a frog. For example, tadpole intestine is a simple tubular structure consisting primarily of a single layer of larval epithelial cells. During metamorphosis, it is transformed into an organ with a multiply folded adult epithelium, which is surrounded by elaborate connective tissue and muscles through specific larval epithelial cell death and de novo development of the adult epithelial stem cells, followed by their proliferation and differentiation. The wealth of knowledge from past research and the ability to manipulate amphibian metamorphosis, both in vivo by using genetic approaches or hormone treatment of whole animals and in vitro in organ cultures, offer an excellent opportunity to 1) study the developmental function of TH receptors (TRs) and the underlying mechanisms in vivo and 2) identify and functionally characterize genes that are critical for organogenesis, particularly for the formation of the adult intestinal epithelial stem cells during postembryonic development in vertebrates. A major recent focus has been to make use of the TALEN and CRISPR/Cas9 technologies to knock out the endogenous genes for functional analyses. In addition, the recent improvements in Xenopus tropicalis genome annotation allow us to carry out RNA-Seq and chromatin-immunoprecipitation (ChIP)-Seq analyses at the genome-wide level. It also allows us to adapt single-cell sequencing technology to study how TH induces cell transformations during vertebrate development. Thus, in recent years, we have focused our research on the diploid Xenopus tropicalis. We complement our frog studies by investigating the genes found to be important for frog intestinal stem-cell development in developing mouse intestine by making use of the ability to carry out conditional knockout.
TR expression and binding to target genes dictate organ-specific functions of thyroid hormone receptors TRα and TRβ during Xenopus metamorphosis.
We generated TRα and TRβ double-knockout animals and observed distinct effects of individual TRα and TRβ knockout on limb development, intestinal remodeling, and tail resorption during development. The effects correlate with the relative expression levels of the TRα and TRβ genes during development in these organs, as previously reported by us and others for both Xenopus laevis and Xenopus tropicalis. More recently, we carried out chromatin immunoprecipitation-sequencing (ChIP-Seq) to identify genes bound by TR in these organs in premetamorphic wild-type and TRα–knockout tadpoles, with or without TH treatment. We found that, at the genome-wide level, the tail had far fewer genes bound by TR or affected by TRα knockout. The findings suggest that the extent of target binding by TR in premetamorphic tadpoles underlies the ability of individual organs to undergo TH–induced metamorphosis given that TH can induce metamorphosis of the intestine and limb but not of the tail of premetamorphic tadpoles, findings that are also consistent with the earlier observation that TRα knockout affects metamorphosis of the limb and intestine but not of the tail. In addition, the extent of target gene binding by TR correlates with the relative TR expression in these organs. Thus, TR expression and its subsequent binding to target genes in a given organ underlie TR function in the organ during Xenopus development.
TR recruits histone-modifying coactivators to regulate gene transcription and metamorphosis.
We previously showed that, during metamorphosis, liganded TR recruits coactivator complexes containing the steroid-receptor coactivator SRC3, a histone acetyltransferase, and the protein arginine methyltransferase 1 (PRMT1), an asymmetric histone arginine methyltransferase, to TH–responsive promoters. To investigate the functions of endogenous SRC3 and PRMT1 during metamorphosis, we generated Xenopus tropicalis animals lacking a functional SRC3 or PRMT1 and analyzed the resulting phenotype. We found that knocking out SRC3 and PRMT1 reduced overall histone acetylation and methylation, respectively, in pre-metamorphic tadpoles. The knockout of either gene does not affect embryogenesis, allowing the development of apparently normal feeding-stage (stage 45) tadpoles by four days of age [Reference 1]. SRC3 knockout tadpoles can complete natural metamorphosis but with delayed intestinal remodeling. In addition, the knockout inhibits TH–induced metamorphosis. We plan further studies to determine the molecular basis of such knockout effects. The PRMT1 knockout, however, led to lethality before the onset of natural metamorphosis. Thus, ongoing studies are directed toward studying the effects of PRMT1 knockout on TH–induced metamorphosis, given that tadpoles as young as the onset of feeding can be induced to undergo most metamorphic changes upon TH treatment.
Simplifying genotyping of mutants from genome editing with parallel qPCR–based iGenotype index
Targeted genome editing is a powerful tool in reverse genetic studies of gene function in many aspects of biological and pathological processes. The CRISPR/Cas system or engineered endonucleases such as ZFNs and TALENs are the most widely used genome-editing tools that are introduced into cells or fertilized eggs to generate double-strand DNA breaks within the targeted region, triggering cellular DNA repair through either homologous recombination or non-homologous end joining (NHEJ). DNA repair through the NHEJ mechanism is usually error-prone, leading to point mutations or indels (insertions and deletions) within the targeted region. Some of the mutations in embryos are germline-transmissible, thus providing an effective way to generate model organisms with targeted gene mutations. However, point mutations and short indels are difficult to effectively genotype, often requiring time-consuming and costly DNA sequencing to obtain reliable results. We developed a parallel qPCR assay in combination with an iGenotype index to allow simple and reliable genotyping [Reference 2]. The genotype-associated iGenotype indexes converged to three simple genotype-specific constant values (1, 0, -1), regardless of allele-specific primers used in the parallel qPCR assays or gene mutations at wide ranges of PCR template concentrations, thus resulting in clear genotype-specific cutoffs, established through statistical analysis, for genotype identification. While we established such a genotyping assay in the Xenopus tropicalis model, the approach should be applicable to genotyping of any organism or cells and can potentially be used for large-scale, automated genotyping.
Liganded TR controls larval epithelial cell death and adult epithelial stem-cell development/proliferation during Xenopus tropicalis metamorphosis.
Studies on individual TR knockout animals have shown that knockout of either TRα or TRβ delays, but does not prevent, intestinal remodeling, including stem-cell formation and/or proliferation. Thus, TRα and TRβ both contribute to and can compensate for the loss of either TR to ensure eventual completion of intestinal remodeling. On the other hand, TR double-knockout (TRDKO) Xenopus tropicalis tadpoles fail to undergo either larval epithelial cell death or adult epithelial stem-cell development and proliferation by stage 61, the climax stage when TRDKO animals die. Thus, liganded TR is essential for larval cell death and adult stem-cell development/proliferation. In addition, knocking out the TR coactivator SRC3 also delays intestinal remodeling, suggesting that liganded TR recruits coactivators such as SRC3 to promote intestinal metamorphosis. We are further investigating the underlying molecular and cellular mechanisms, particularly with single-cell RNA-Seq (scRNA-Seq), to determine TH regulation of the changes within different types of cells in the intestine during metamorphosis.
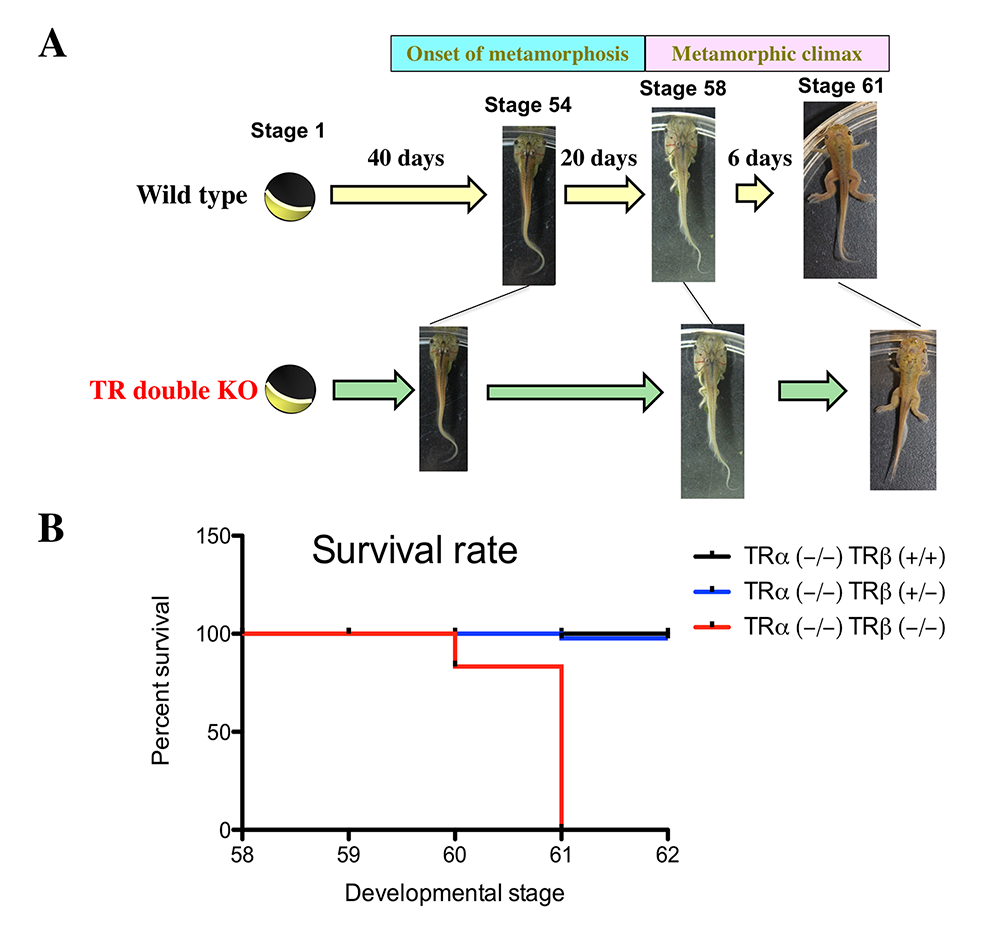
Figure 1. Effects of TR double KO on developmental rate: TR double KO leads to premature initiation of metamorphosis but slows metamorphic progress and causes lethality at the metamorphic climax.
A. TR double KO animals take a shorter time to reach the onset of metamorphosis (stage 54), indicating accelerated pre-metamorphic development. Once metamorphosis begins, the KO animals take longer to reach the beginning of metamorphic climax (stage 58) and also develop more slowly during the climax stages, between stages 58 and 61. The length of each stage indicates the relative time needed for development between two adjacent stages.
B. Tadpoles without any TR die during the climax of metamorphosis. The tadpoles of mixed genotypes at stage 58 were able to develop to stage 62 and were genotyped at stage 62 or when they died during this developmental period. The survival rate for each of the three genotypes, trα–/–trβ+/+, trα–/–trβ+/–, and trα–/–trβ–/–, was thus obtained and plotted. Note that no double KO tadpoles developed to stage 62 but that a single copy of trβ+/– was sufficient for the animal to complete metamorphosis and develop into a reproductive adult.
Figure 1. Effects of TR double KO on developmental rate: TR double KO leads to premature initiation of metamorphosis but slows metamorphic progress and causes lethality at the metamorphic climax.
A. TR double KO animals take a shorter time to reach the onset of metamorphosis (stage 54), indicating accelerated pre-metamorphic development. Once metamorphosis begins, the KO animals take longer to reach the beginning of metamorphic climax (stage 58) and also develop more slowly during the climax stages, between stages 58 and 61. The length of each stage indicates the relative time needed for development between two adjacent stages.
B. Tadpoles without any TR die during the climax of metamorphosis. The tadpoles of mixed genotypes at stage 58 were able to develop to stage 62 and were genotyped at stage 62 or when they died during this developmental period. The survival rate for each of the three genotypes, trα–/–trβ+/+, trα–/–trβ+/–, and trα–/–trβ–/–, was thus obtained and plotted. Note that no double KO tadpoles developed to stage 62 but that a single copy of trβ+/– was sufficient for the animal to complete metamorphosis and develop into a reproductive adult.
Protein arginine methyltransferase 1 regulates epithelial homeostasis in adult mouse intestine.
Adult stem cells play an essential role in adult organ physiology and tissue repair and regeneration. While much has been learnt about the property and function of various adult stem cells, the mechanisms of their development remain poorly understood in mammals. Earlier studies suggested that the formation of adult mouse intestinal stem cells takes place during the first few weeks after birth, the postembryonic period when plasma TH levels are high. Furthermore, deficiency in TH signaling leads to defects in adult mouse intestine, including reduced cell proliferation in the intestinal crypts, where stem cells reside. Our earlier studies showed that protein arginine methyltransferase 1 (PRMT1), a TR coactivator, is highly expressed during intestinal maturation in the mouse, resembling its upregulation during intestinal metamorphosis. Furthermore, we previously showed that PRMT1 is important for adult intestinal stem-cell development and/or proliferation during Xenopus metamorphosis. To determine whether PRMT1 has a conserved role in adult stem-cell development, we analyzed the expression of PRMT1 by immunohistochemistry and studied the effect of tissue-specific knockout of PRMT1 in the intestinal epithelium. We showed that PRMT1 is highly expressed in the proliferating transit-amplifying cells and crypt-base stem cells. Furthermore, we showed that specific knockout of PRMT1 in the intestinal epithelium resulted in, surprisingly, more elongated adult intestinal crypts with increased cell proliferation in the adult mice. In addition, there were more Goblet cells and fewer Paneth cells in the crypt. To investigate whether the knockout phenotype is attributable to the role of PRMT1 during intestinal development or adult intestinal homeostasis, we further generated a tamoxifen-inducible intestinal epithelium–specific PRMT1 knockout mouse model and found that tamoxifen-induced knockout of PRMT1 in adult mice resulted in the same intestinal phenotypes [Reference 3]. Thus, for the first time our studies revealed that the epigenetic enzyme PRMT1 is important for the maintenance of intestinal epithelial architecture and homeostasis, although PRMT1 may also influence intestinal development.
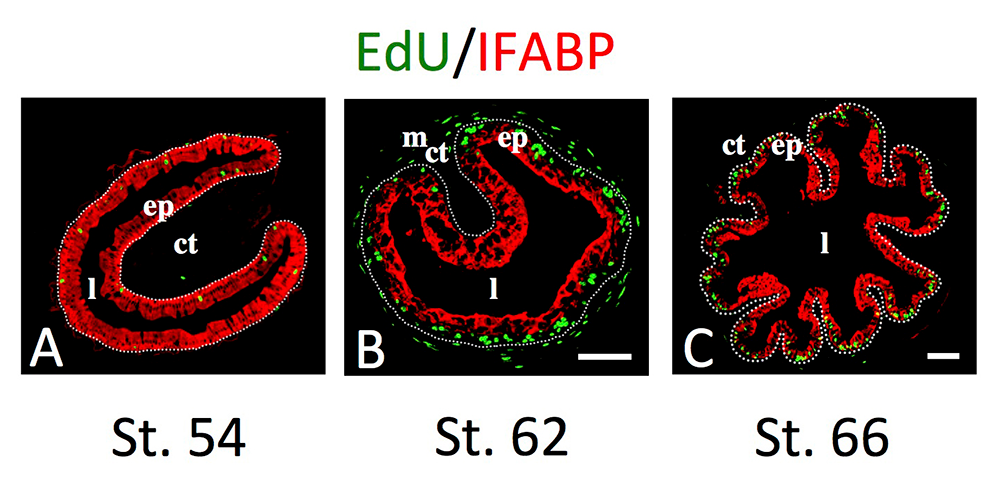
Figure 2. Intestinal metamorphosis involves the formation of clusters of proliferating, undifferentiated epithelial cells at the climax.
Tadpoles at pre-metamorphic stage 54 (A), climax, stage 62 (B), and the end of metamorphosis, stage 66 (C) were injected with 5-ethynyl-2′-deoxyuridine (EdU) one hour before sacrifice. Cross-sections of the intestine from the resulting tadpoles were double-stained by EdU labeling of newly synthesized DNA and by immunohistochemistry of IFABP (intestinal fatty acid–binding protein), a marker for differentiated epithelial cells. The dotted lines depict the epithelium-mesenchyme boundary. Note that there are few EdU–labeled proliferating cells in the epithelium and that they express IFABP at pre-metamorphosis (A) and increase in the form of clustered cells (proliferating adult stem cells), which lack IFABP at the climax of metamorphosis (B). At the end of metamorphosis, EdU–labeled proliferating cells are localized mainly in the troughs of the epithelial folds, where IFABP expression is low (C). ep, epithelium; ct, connective tissue; m, muscles; l, lumen.
Figure 2. Intestinal metamorphosis involves the formation of clusters of proliferating, undifferentiated epithelial cells at the climax.
Tadpoles at pre-metamorphic stage 54 (A), climax, stage 62 (B), and the end of metamorphosis, stage 66 (C) were injected with 5-ethynyl-2′-deoxyuridine (EdU) one hour before sacrifice. Cross-sections of the intestine from the resulting tadpoles were double-stained by EdU labeling of newly synthesized DNA and by immunohistochemistry of IFABP (intestinal fatty acid–binding protein), a marker for differentiated epithelial cells. The dotted lines depict the epithelium-mesenchyme boundary. Note that there are few EdU–labeled proliferating cells in the epithelium and that they express IFABP at pre-metamorphosis (A) and increase in the form of clustered cells (proliferating adult stem cells), which lack IFABP at the climax of metamorphosis (B). At the end of metamorphosis, EdU–labeled proliferating cells are localized mainly in the troughs of the epithelial folds, where IFABP expression is low (C). ep, epithelium; ct, connective tissue; m, muscles; l, lumen.
Protein arginine methyltransferase 1 regulates mouse enteroendocrine cell development and homeostasis.
To investigate how PRMT1 affects adult intestinal epithelial homeostasis, we performed RNA-Seq on small intestinal crypts of tamoxifen-induced intestinal epithelium–specific PRMT1 knockout and wild-type adult mice. We found that wild-type and PRMT1–deficient small intestinal crypts exhibited markedly different mRNA profiles [Reference 4]. Surprisingly, GO (gene ontology) terms and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analyses showed that the most significantly enriched pathways among the genes upregulated in PRMT1 knockout crypts were associated with enteroendocrine cells (EECs), which are one type of intestinal secretory cell and are the most abundant hormone-producing cells involved in the control of energy homeostasis in mammals. We found that genes encoding enteroendocrine-specific hormones and transcription factors were upregulated in PRMT1–deficient small intestine. Moreover, we found a marked increase in the number of EECs in the PRMT1–knockout small intestine. Concomitantly, there were more Neurogenin 3–positive enteroendocrine progenitor cells in the small intestinal crypts of the knockout mice, accompanied by the upregulation of the expression levels of downstream targets of Neurogenin 3, including Neuod1, Pax4, Insm1, in PRMT1–deficient crypts. For the first time, our findings revealed that the epigenetic enzyme PRMT1 controls mouse enteroendocrine cell development, most likely via inhibition of Neurogenin 3–mediated commitment to EEC lineage, further suggesting a potential role of PRMT1 as a critical transcriptional cofactor in EEC specification and homeostasis to affect metabolism and metabolic diseases.
L-type amino acid transporter 1 (LAT1) regulates cell proliferation and secretary cell differentiation and distribution in adult mouse intestine.
To regulate cellular processes, TH has to be actively transported into cells, a process that is mediated by several different types of transporter. One of our previously identified TH–response genes in Xenopus intestine, LAT1, encodes the light chain of a heterodimeric system L type of TH transporter, which also transports several amino acids to activate mTORC1 signaling. Interestingly, LAT1 is highly upregulated at the climax of metamorphosis in tadpole intestine, coinciding with the formation and rapid proliferation of adult intestinal stem cells. We also found that LAT1 was also highly expressed in the mouse intestine during the neonatal period when mouse intestine matures into the adult form, a process that appears also to involve TH–dependent formation and/proliferation of adult intestinal stem cells. In a collaborative study, we generated a mouse line with the LAT1 gene floxed, which allows conditional knockout of LAT1 upon expression of the Cre recombinase. By using villin-promoter to drive Cre expression, we generated intestinal epithelial cell–specific LAT1 knockout (LAT1ΔIEC) [Reference 5]. We showed that LAT1 is highly expressed in mouse intestinal crypt and that LAT1ΔIEC reduces mTORC1 signaling. Surprisingly, in adult LAT1ΔIEC intestinal crypts cell proliferation is elevated but there are fewer mature Paneth cells. Goblet cells, the other major secretory cell type in the small intestine, are increased in the crypts but reduced in the villi. Analyses with scRNA-Seq and electron microscopy revealed dedifferentiation of Paneth cells in LAT1ΔIEC mice, leading to markedly reduced secretory granules, with little effect on Paneth cell number. Thus, LAT1 likely regulates secretory-cell differentiation to affect stem-cell niche and indirectly regulate cell proliferation.
Additional Funding
- FY23 NICHD Early Career Award for Lingyu Bao
Publications
- Steroid-receptor coactivator complexes in thyroid hormone-regulation of Xenopus metamorphosis. Vitam Horm 2023 123:483–502
- Simplifying genotyping of mutants from genome editing with parallel qPCR-based iGenotype index. Cells 2024 13:247
- Protein arginine methyltransferase 1 is required for the maintenance of adult small intestinal and colonic epithelial cell homeostasis. Int J Biol Sci 2024 20:554–568
- Protein arginine methyltransferase 1 regulates mouse enteroendocrine cell development and homeostasis. Cell Biosci 2024 14:70
- Amino acid transporter SLC7A5 regulates cell proliferation and secretary cell differentiation and distribution in the mouse intestine. Int J Biol Sci 2024 20:2187–2201
Collaborators
- Ryan Dale, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
- Caroline Esnault, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
- James Iben, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Tianwei Li, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Vivek Mahadevan, PhD, Molecular Genomics Core, NICHD, Bethesda
- Fabio Rueda Faucz, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Laurent Sachs, PhD, CNRS, Paris, France
- Bingyin Shi, MD, Xi’an Jiaotong University School of Medicine, Xi'an, China
- Guihong Sun, PhD, Wuhan University School of Medicine, Wuhan, China
- Chuan Wu, MD, PhD, Experimental Immunology Branch, NCI, Bethesda, MD
- Henry Zhang, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
Contact
For more information, email shi@helix.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/shi.