Point of Care and Wearable Biophotonics for Characterizing Tissue Composition and Metabolism
- Bruce Tromberg,
PhD, Head, Section on Biomedical Optics, Director of the National Institute of Biomedical Imaging and Bioengineering - Timothy Quang, PhD, Staff Scientist
- Brian Hill, MS, Biomedical Engineer
- Ola Abdalsalam, PhD, Postdoctoral Fellow
- Helen Parker, PhD, Postdoctoral Fellow
- Eric Gallagher, BS, Postbaccalaureate Fellow
- Young Kim, BS, Postbaccalaureate Fellow
- Golnar Mostashari, BS, Postbaccalaureate Fellow
- Emily Yu, BS, Postbaccalaureate Fellow
- Andrew Ferrer, BS, Summer Student
- Alexander Le, Summer Student
- Ali Owji, BS, Summer Student

By advancing models, methods, and devices that utilize the interaction of light with biological tissue, we strive to develop non-invasive techniques that can help guide therapy and aid in clinical decision-making. The techniques are used to perform real-time quantitative measurements of clinically relevant information, including tissue blood flow, oxygen extraction, and body/tissue composition. Our research seeks to move such technologies from ‘bench to bedside,’ where they can be applied to clinical problems, including vascular and metabolic diseases.
Optical characterization of vascular health in sickle cell disease
Sickle cell disease (SCD) is an inherited hemoglobinopathy that disproportionately impacts minority populations in the United States and affects about 100,000 individuals. The substitution of valine by glutamic acid on the 6th amino acid of beta globin results in an abnormal hemoglobin variant (HbS), which polymerizes once deoxygenated and alters both the structure and function of red blood cells (RBCs), distorting them into a ‘sickle’ shape. Sickled RBCs have one sixth of the lifespan of normal RBCs, resulting in chronic hemolytic anemia; they are also rigid and can obstruct microvascular blood flow, causing recurring, unpredictable cycles of acute vaso-occlusive pain, commonly referred to as vaso-occlusive crises (VOC). Recurrent VOCs lead to hypoxia-reperfusion injury and, together with the intravascular hemolysis, promote inflammation and vascular endothelial damage. The systemic vasculopathy affects many organs, leading to cardiovascular complications, chronic pain, and cerebral and kidney impairment. Because current treatment options are not universally effective, there is a significant, unmet need for new technologies that can quantitatively characterize SCD physiology and provide new insights for optimizing therapeutic impact in SCD patients.
Given the impairments to microvascular flow and endothelial dysfunction associated with SCD, advanced quantitative NIRS (near-infrared spectroscopy) is an attractive candidate to provide comprehensive hemodynamic evaluations in point-of-care settings. We are optically characterizing tissue composition, metabolism, and perfusion in several SCD studies, led by our collaborators Swee Lay Thein, Arun Shet, and Courtney Fitzhugh.
NCT04610866 – Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Long-term Mitapivat Dosing in Subjects With Stable Sickle Cell Disease: An Extension of a Phase I Pilot Study of Mitapivat
NCT05675436 – Observational Study Investigating the Mechanistic Effects of Mitapivat in Subjects with Sickle Cell Disease
NCT05604547 – Exploring Near Infrared Spectroscopy (NIRS) Technologies for Assessment of Muscle Physiology, Tissue Oxygenation, and Blood Flow in Patients With Sickle Cell Disease (SCD)
NCT04514510 – Fixed Dose Flavonoid Isoquercetin on Thrombo-Inflammatory Biomarkers in Subjects With Stable Sickle Cell Disease
NCT05213572 – Observational Study to Deeply Phenotype Major Organs in Sickle Cell Disease After Curative Therapies
Our group's role is to assess the sensitivity of various optical devices to hemodynamic changes induced by SCD treatments and to evaluate whether the changes correlate with what is observed from blood chemistry. Findings published this year are illustrated in Figure 1.
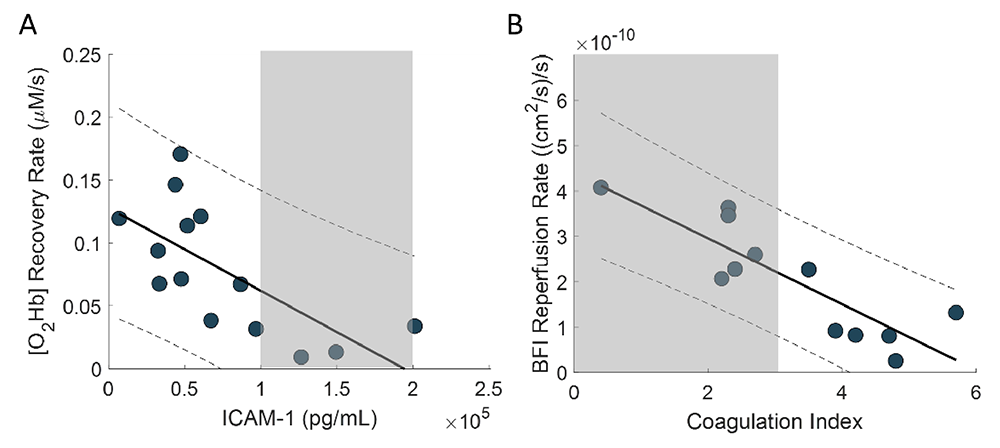
Figure 1. Correlations between optical skeletal muscle hemodynamics and hematology biomarkers
Gray areas represent the healthy range of values for each hematology marker.
A. Spearman’s correlation coefficient between [O2Hb] recovery rate and ICAM-1 (intercellular adhesion molecule 1) was –0.75 (p = 0.003) at baseline.
B. Spearman’s correlation coefficient between BFI (blood flow index) reperfusion rate vs. CI (coagulation index) was –0.82 for baseline (p = 0.001). In patients with SCD, elevated ICAM-1 levels and coagulation index are the result of the chronic ischemia-reperfusion injury promoted by continuous cycles of RBC sickling and increased ‘stickiness’ of RBC's, respectively. Such increased clinical measures are associated with increased cardiovascular risk and endothelial dysfunction, which appear to be mirrored by a decrease in our optical markers describing vascular reactivity.
Figure 1. Correlations between optical skeletal muscle hemodynamics and hematology biomarkers
Gray areas represent the healthy range of values for each hematology marker.
A. Spearman’s correlation coefficient between [O2Hb] recovery rate and ICAM-1 (intercellular adhesion molecule 1) was –0.75 (p = 0.003) at baseline.
B. Spearman’s correlation coefficient between BFI (blood flow index) reperfusion rate vs. CI (coagulation index) was –0.82 for baseline (p = 0.001). In patients with SCD, elevated ICAM-1 levels and coagulation index are the result of the chronic ischemia-reperfusion injury promoted by continuous cycles of RBC sickling and increased ‘stickiness’ of RBC's, respectively. Such increased clinical measures are associated with increased cardiovascular risk and endothelial dysfunction, which appear to be mirrored by a decrease in our optical markers describing vascular reactivity.
Development of a wearable point-of-care monitoring device for pediatric obstructive sleep apnea
Obstructive sleep apnea (OSA) is the most common type of sleep apnea, in which the blockage of the airway causes the patient to stop breathing involuntarily for ten seconds or more throughout the night during sleep. When breathing stops, the oxygen level in the blood falls, sometimes waking the sleeper. Pediatric obstructive sleep apnea (POSA) can be particularly concerning, with several associated morbidities, which can have long-term effects extending into adulthood, including adverse changes in cardiovascular, metabolic, and developmental health. Onset usually occurs between ages 2–8, as tonsils reach their peak growth. Unfortunately, pediatric OSA remains largely under-diagnosed because of a lack of education about symptoms and the limited availability of sleep-medicine physicians. Early diagnosis and treatment are imperative for preventing many of these morbidities.
The non-invasive technology NIRS (near-infrared spectroscopy) is well suited for measuring cerebral hemodynamics during sleep. NIRS uses near-infrared light to interrogate human tissue and measure changes in oxygenation. Extensive literature documents the utility of NIRS in sleep studies of both normal and sleep-disordered breathing (SDB). In normal sleep, NIRS has been used to investigate cerebral hemodynamics during sleep stages and transitions between stages. For sleep apnea applications, NIRS can detect reductions in cerebral oxygenation, as well as respiration, heart rate, and blood flow changes that occur as the result of apnea. Previous work in NIRS sleep analysis showed that transient rises in cerebral deoxyhemoglobin are a prominent feature of apneas and hypopneas, and it has been shown that autoregulatory mechanisms fail to prevent hypoxia during severe obstructive events. NIRS is uniquely suited to capture such breathing-induced cerebrovascular disturbances with high sensitivity and temporal resolution.
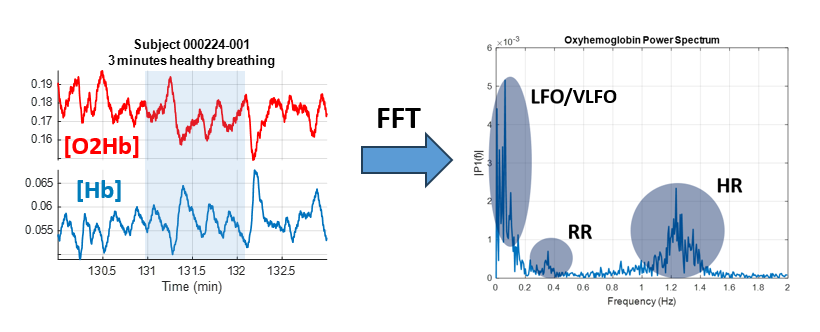
Figure 3. Frequency content of healthy breathing during sleep
Changes in oxyhemoglobin and deoxyhemoglobin (left) measured by near-infrared spectroscopy. A fast fourier transform (FFT) allows for display of the frequency content of the signal (right). In addition to sensitivity to heart and respiratory rate, low frequency components are seen in the range of .05–.15 Hz. The power as well as the relationship in time between oxyhemoglobin and deoxyhemoglobin signals in different frequency bands can characterize underlying physiological processes and help identify signal origins.
Figure 3. Frequency content of healthy breathing during sleep
Changes in oxyhemoglobin and deoxyhemoglobin (left) measured by near-infrared spectroscopy. A fast fourier transform (FFT) allows for display of the frequency content of the signal (right). In addition to sensitivity to heart and respiratory rate, low frequency components are seen in the range of .05–.15 Hz. The power as well as the relationship in time between oxyhemoglobin and deoxyhemoglobin signals in different frequency bands can characterize underlying physiological processes and help identify signal origins.
NCT05052216 – Development of a Wearable Point of Care Monitoring Device for Pediatric Obstructive Sleep Apnea
In collaboration with Ashura Buckley and colleagues, we are currently enrolling children aged 3–12 to investigate how optical signals change throughout sleep by comparing physiological signals derived from NIRS with data from polysomnography. More than 60 children have participated in this study to date, and data analysis is ongoing.
Vascular diseases driven by genetic alterations and COVID infection
The study and management of diseases with unknown vascular phenotypes is challenging. Given the importance of the vascular network to every organ system, understanding the extent and severity of vascular complications is necessary in order to develop effective treatments. Monogenic vascular diseases are characterized by a single genetic mutation, which can have deleterious effects on protein structure, function, or synthesis, and can be associated with severe complications, with high mortality and morbidity rates. While each individual disease is rare, these diseases can encompass a wide array of vascular phenotypes affecting any part of the body. Infectious diseases are another pathway for vascular complications, which can either manifest directly by viral/bacterial infection of the endothelial lining or indirectly as a result of damage triggered by inflammatory responses to the pathogen.
For this project, we aim to develop multi-modal NIRS assessment methods that are sensitive to the hemodynamic impairments of two patient cohorts: (1) patients with rare monogenic vascular disease; and (2) patients recovering from COVID-19. This consists of the selection of both the appropriate optical modality and measurement challenge to characterize the afflicted area. Our goal is to perform comprehensive clinical evaluations of patients recruited into the study, in order to better understand the disease pathology, heterogeneity of symptoms within the disease population, and progression of the various, rare vascular diseases over time. In collaboration with Manfred Boehm, three specific disease cohorts will be explored: ACDC (arterial calcification due to deficiency in the enzyme CD73), CADASIL (cerebral autosomal dominant arteriopathy and leukoencephalopathy), and COVID-19.
NCT03538639 – Vascular Disease Discovery Protocol
ACDC is characterized by progressive vascular calcification, typically affecting arteries of the lower limbs, and manifests clinically as debilitating lower extremity pain resulting from the lower limb claudication and ischemia. For the ACDC cohort, we will evaluate vascular reactivity and endothelial function in the lower limbs using a hyperthermia challenge with an optical probe combining diffuse reflectance spectroscopy (DRS) and laser doppler flowmetry (LDF) (Perimed AB, Sweden) to evaluate vascular reactivity in skin. The probe measures hemodynamic parameters such as hemoglobin (Hb) concentration, tissue oxygen saturation (StO2), and tissue perfusion. Measures of vascular reactivity and endothelial function in the lower limbs can provide a rapid and direct method for assessing the extent of severity.
NCT05072483 – Natural History Study of CADASIL
CADASIL is a monogenic small-vessel disease that affects the arteries in the brain. Clinical manifestations include migraines, recurrent strokes, and progressive white matter degeneration. Current diagnosis for CADASIL is through MRI imaging of the white matter, genetic testing, or through family history. Previous literature reported impaired cerebrovascular reactivity detected by MRI and transcranial doppler. NIRS is an attractive alternative method for assessing cerebrovascular reactivity because of its compact size and penetration depth. For the CADASIL cohort, optical probes will be affixed to the forehead and forearm to acquire information from the prefrontal cortex and skeletal muscle respectively. The optical measurement consists of a five-minute baseline period followed by a five-minute brachial cuff occlusion and a five-minute recovery period. A series of three end-exhalation breath holds follow the recovery period; patients will be asked to exhale and hold their breaths for either 30 seconds or for as long as they can tolerate. We anticipate that these assessments of cerebrovascular compared with skeletal muscle reactivity could provide a predictive test of disease progression.
NCT04595773 - COVID-19 - Chronic Adaptation and Response to Exercise (COVID-CARE): a Randomized Controlled Trial
While COVID-19 is mainly perceived as a respiratory illness, there is also significant evidence of vascular complications, especially in those with severe symptoms and long-term effects. In collaboration with Manfred Boehm's and Leighton Chan's groups, we performed optical hemodynamic assessments in a cohort of patients recovering from COVID. The measurements are one component of a larger randomized clinical study evaluating the efficacy of targeted exercise intervention to improve recovery from COVID. Patients recruited for this study are randomized into either a control or treatment arm. Patients in the treatment arm undergo a ten-week exercise regimen after their baseline visit, followed by a follow-up visit. Patients in the control arm continue with their typical daily routine for ten weeks after the baseline visit and return for a follow-up visit; patients then begin the ten-week exercise regimen followed by another follow-up visit. Patients recruited into the study undergo a brachial cuff occlusion and also participate in a set of breath-holding exercises. In a separate measurement session, patients undergo an exercise test during which optical measures are acquired before and after exercise. As the study is ongoing, analysis is currently limited to comparing optical measures to established clinical metrics. A more comprehensive analysis will be performed upon conclusion of the study.
Continuous blood-pressure and vascular monitoring technologies for optimizing maternal health
Pre-eclampsia is a pregnancy-specific disorder responsible for more than 70,000 maternal deaths and 500,000 fetal deaths worldwide every year. While high blood pressure is the main clinical phenotype, pre-eclampsia can trigger several hemodynamic changes, including increased vascular stiffness and reduced vascular reactivity. While delivery can resolve most symptoms, they can persist postpartum and continue to impact the mother. Early diagnosis combined with treatment remains key to improving patient outcomes. However, the transition from gestational hypertension to mild pre-eclampsia to severe pre-eclampsia is a dynamic process; early diagnosis would ideally necessitate continuous, heightened surveillance. There is a need for technologies that can easily monitor the hemodynamic state of the mother and aid in the detection and risk stratification of pre-eclampsia.
Affixed transmission speckle analysis (ATSA) is a promising candidate to measure blood flow, vascular stiffness, and pulse transit time in a compact form-factor. To recover blood flow information, ATSA sends coherent light through a peripheral digit to measure intensity fluctuations caused by moving red blood cells. The high acquisition speed of ATSA enables the recovery of a pulsatile waveform originating from blood flow, referred to as the speckle plethysmograph (SPG). While previous work with pulse transit time evaluated the feasibility of PPG–based approaches, the SPG offers better signal quality than the photoplethysmograph (PPG), with SPG estimations of heart rate variability having improved accuracy over PPG estimations.
NCT05554315 - Speckle Plethysmography Pulse Transit Time as a Marker of Blood Pressure Changes
This healthy volunteer protocol seeks to investigate ATSA/SPG for cuff-free blood pressure estimation. Participants are asked to undergo a number of challenges designed to perturb blood pressure while being monitored by PPG and SPG sensors alongside ECG and a continuous noninvasive arterial pressure monitor.
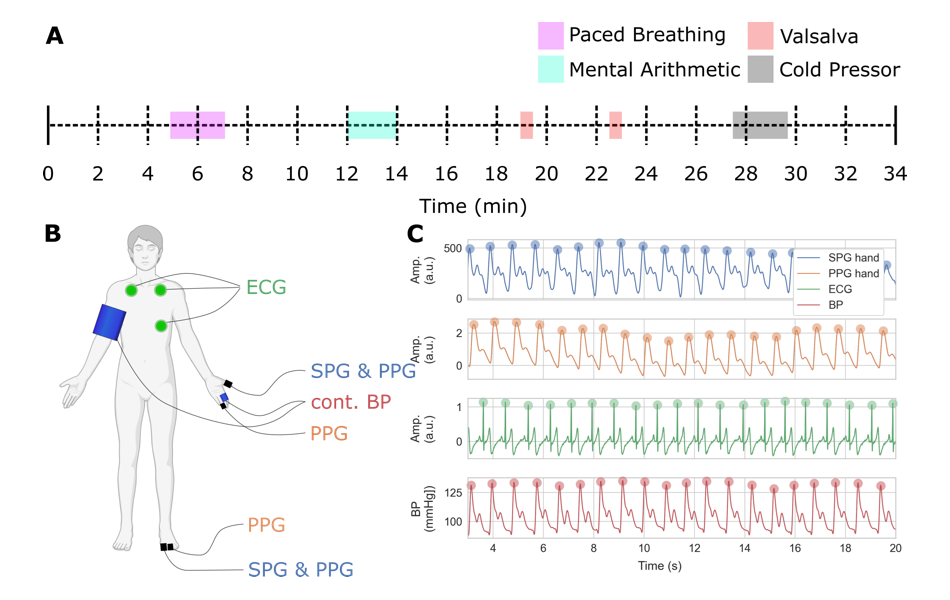
Figure 2. SPG cuff-free blood pressure estimation: study design
A. Timeline of measurement including sections of paced breathing, mental arithmetic, Valsalva maneuver (forceful attempt of exhalation against a closed airway), and cold pressor, separated by periods of rest.
B. Location of non-invasive sensors including SPG, PPG, continuous blood pressure, and ECG (partially created with BioRender.com). C. Example of subset of recorded data showing the SPG, PPG, BP, and ECG co-registered waveforms per cardiac cycle.
Figure 2. SPG cuff-free blood pressure estimation: study design
A. Timeline of measurement including sections of paced breathing, mental arithmetic, Valsalva maneuver (forceful attempt of exhalation against a closed airway), and cold pressor, separated by periods of rest.
B. Location of non-invasive sensors including SPG, PPG, continuous blood pressure, and ECG (partially created with BioRender.com). C. Example of subset of recorded data showing the SPG, PPG, BP, and ECG co-registered waveforms per cardiac cycle.
Optical techniques for the evaluation of brain metabolism in neurocognitive disorders
Neurocognitive diseases, which can have devastating effects, often affect how the brain uses oxygen. While imaging techniques capable of assessing cerebral blood flow and metabolism, such as FDG-PET (18-fluorodeoxyglucose positron emission tomography), have been used to evaluate disease severity and monitor progression, these modalities are resource-intensive and may use radiation, which make them unsuitable for researching these disorders in children. Given these limitations, there is a paucity of data regarding brain metabolic function in childhood neurocognitive disorders. New methods are needed to non-invasively characterize these disorders and improve evaluation of disease severity, progression, and therapy monitoring.
Diffuse correlation spectroscopy (DCS) is an emerging non-invasive optical technique that uses the light fluctuations of moving particles to quantify blood flow deep in tissue. When coupled with advanced NIRS methods that characterize tissue absorption and oxygenation, the total metabolic rate of oxygen consumption can be recovered. In collaboration with Forbes Porter, Samar Rahhal, and Amir Gandjbakhche, we are exploring the feasibility of using NIRS and DCS to provide a non-invasive, non-sedative method for functional brain imaging of neurocognitive disorders. A pilot study was launched this year that uses these technologies to study a number of disorders that are extensively followed by NICHD clinicians: Niemann-Pick disease type C1 (NPC1), Smith Lemli Opitz syndrome (SLOS), and Juvenile Neuronal Ceroid Lipofuscinosis (CLN3).
NCT05642221 - Pilot Study of the Use of Functional Near-Infrared Spectroscopy (fNIRS) Combined with Diffuse Correlation Spectroscopy (DCS) in Neurocognitive Disease as Compared to Healthy Neurotypical Controls.
To date, over 70 subjects have been enrolled in this study, and we are currently finalizing analysis of the DCS data.
Publications
- Non-invasive optical and laboratory hematologic biomarkers correlate in patients with sickle cell disease. Biomed Opt Express 2024 15(8):4829–4841
- Incorporating point-of-care technologies to assess treatment response in sickle cell disease. Blood Vessels, Thrombosis & Hemostasis 2024 1(2):ISSN 2950–3272
Collaborators
- Manfred Boehm, MD, Laboratory of Cardiovascular Regenerative Medicine, NHLBI, Bethesda, MD
- Ashura Buckley, MD, Office of the Clinical Director, NIMH, Bethesda, MD
- Leighton Chan, MD, MPH, Rehabilitation Medicine, Clinical Center, NIH, Bethesda, MD
- Courtney Fitzhugh, MD, Cellular and Molecular Therapeutics Branch, NHLBI, Bethesda, MD
- Amir Gandjbakhche, PhD, Section on Translational Biophotonics, NICHD, Bethesda, MD
- Thomas J. Pohida, MS, Instrumentation Development and Engineering Application Solutions, NIBIB, Bethesda, MD
- Forbes Porter, MD, Office of the Clinical Director, NICHD, Bethesda, MD
- Randall Pursley, Instrumentation Development and Engineering Application Solutions, NIBIB, Bethesda, MD
- Samar Rahhal, MD, Office of the Clinical Director, NICHD, Bethesda, MD
- Roberto Romero, MD, D(Med)Sci, Perinatology Research Branch, NICHD, Detroit, MI
- Arun Shet, MD, PhD, Sickle Cell Branch, NHLBI, Bethesda, MD
- Hiroaki Suzuki, PhD, Hamamatsu Photonics K.K., Hamamatsu, Japan
- Swee Lay Thein, MB, FRCP, FRCPath, DSc, Sickle Cell Branch, NHLBI, Bethesda, MD
Contact
For more information, email bruce.tromberg@nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/tromberg.