Organ and Tissue Formation during Development
- Brant M. Weinstein,
PhD, Head, Section on Vertebrate Organogenesis - Miranda Marvel, PhD, Staff Scientist
- Daniel Castranova, MS, Scientific Technician
- Van Pham, BS, Scientific Technician
- Leah Greenspan, PhD, Postdoctoral Fellow
- Kanako Inoue, PhD, Postdoctoral Fellow
- Jian Ming Khor, PhD, Postdoctoral Fellow
- Aurora Kraus, PhD, Postdoctoral Fellow
- Jong Park, PhD, Postdoctoral Fellow
- Kiyohito Taimatsu, PhD, Postdoctoral Fellow
- Vishakha Vishwakarma, PhD, Postdoctoral Fellow
- Megan Detels, BS, Graduate Student
- Charles White, BS, Graduate Student
- Isabella Cisneros, BS, Postbaccalaureate Fellow
- Justin Gutkowski, BS, Postbaccalaureate Fellow
- Addison Parker, BS, Postbaccalaureate Fellow
- Jean Prosper Santiago, BS, Postbaccalaureate Fellow
- Zoe Zwick, BS, Postbaccalaureate Fellow

The major focus of the Section is to understand how the elaborate networks of blood and lymphatic vessels arise during vertebrate development. Blood vessels supply every tissue and organ with oxygen, nutrients, and cellular and humoral factors. Lymphatic vessels drain fluids and macromolecules from the interstitial spaces of tissues, returning them to the blood circulation, and they play an important role in immune responses. Our studies on the formation of blood and lymphatic vessels are of great clinical interest because of the roles that both types of vessel play in pathologies such as cancer and ischemia.
The zebrafish (Danio rerio), a small tropical freshwater fish, possesses a unique combination of features that make it particularly suitable for studying vessel formation. Zebrafish are genetically tractable vertebrates with externally developing, optically clear embryos, which are readily accessible to observation and experimental manipulation, features that permit observation of every vessel in the living animal and simple, rapid screening for even subtle vascular-specific defects (Figure 1). Our current studies use genetic screening, experimental analysis, and imaging to examine cues directing vascular growth, patterning, and morphogenesis, regulation of vascular integrity, assembly of the lymphatic system, and the roles of novel vascular-associated cells.
Figure 1. The zebrafish vascular system
Confocal micro-angiogram of the vascular system of a 4½-day-old zebrafish larva labeled by injecting fluorescent microspheres. The transparency of zebrafish larvae makes it possible to use high-resolution optical imaging methods to visualize the entire vasculature in exquisite detail.
Figure 1. The zebrafish vascular system
Confocal micro-angiogram of the vascular system of a 4½-day-old zebrafish larva labeled by injecting fluorescent microspheres. The transparency of zebrafish larvae makes it possible to use high-resolution optical imaging methods to visualize the entire vasculature in exquisite detail.
In addition to our work on vessel development, as a second major effort, we are pursuing studies on the role of epigenetics during early development, in particular how DNA methylation and other epigenetic mechanisms help coordinate cell, tissue, and organ specification and differentiation during development and regeneration, using a novel “EpiTag” epigenetic reporter line and genetic screens for tissue-specific vertebrate epigenetic regulators.
Specification and patterning of developing blood vessels
We are working to elucidate the cellular and molecular mechanisms responsible for the specification, patterning, and differentiation of blood vessels during development. Blood vessels are ubiquitous and vital components of vertebrate animals, innervating and supplying every tissue and organ with oxygen and nutrients. Many of the recent insights into mechanisms of blood vessel formation have come from studies in model organisms, including the zebrafish. We are carrying out several projects using fish, described below.
New tools for experimental analysis of vascular development
We generate novel transgenic lines for visualizing different types of endothelial and perivascular cells, and for driving gene expression or performing molecular profiling of mRNAs in these cell populations.
Genetic analysis of vascular development
We have identified novel mutants affecting vascular development in our transgene-assisted forward-genetic screens and are characterizing the phenotypes and molecular basis for several of the mutants.
Analysis of vascular specification, patterning, morphogenesis, and function
We are studying the development and function of several vascular beds, including the vasculature of the gills, the fish equivalent of the mammalian lungs, which contains unique endothelial cell populations with important roles in gas exchange.
Regulation of vascular integrity and vascular repair
We are using the zebrafish to understand the cellular and molecular mechanisms responsible for proper vessel morphogenesis, for the generation and maintenance of vascular integrity, and for proper repair of the vasculature after injury. Disruption of vascular integrity is associated with hemorrhagic stroke, a severe and debilitating form of stroke associated with high morbidity and mortality. Meningeal vascular dysfunction is also associated with neuro-cognitive deficits and neurodegenerative disease. Vessels play important roles in supporting wound healing and recovery and regeneration of tissues and organs after injury, including the skin. Many recent insights into molecular mechanisms regulating the vasculature in health and disease have come from studies in model organisms such as the zebrafish. We are pursuing several related projects.
Genes regulating vascular integrity
We used forward-genetic screens to identify mutants that disrupt cranial vascular integrity in the zebrafish (Figure 2), and next-gen sequencing methods to identify the defective genes from these mutants, including GDF6 (encoding growth differentiation factor 6,) and RHOA (encoding a small GTPase protein), uncovering their roles in vascular integrity and angiogenesis.
Figure 2. Intracranial hemorrhage (ICH) in the developing zebrafish
The clarity of zebrafish larvae also makes it straightforward to screen for animals with intracranial hemorrhage, as is evident in comparing lateral views of a 2-day-old wild-type larva (A) with a hemorrhage-prone larva deficient in the gene rap1b (B).
Figure 2. Intracranial hemorrhage (ICH) in the developing zebrafish
The clarity of zebrafish larvae also makes it straightforward to screen for animals with intracranial hemorrhage, as is evident in comparing lateral views of a 2-day-old wild-type larva (A) with a hemorrhage-prone larva deficient in the gene rap1b (B).
Re-vascularization after cutaneous injury
Vascular regrowth and remodeling are critical for proper wound healing, and defective vessel growth in cutaneous wounds is associated with delayed wound repair and/or chronic open wounds that are susceptible to infection. We have developed a new zebrafish model of vascular regrowth and repair after cutaneous injury in adult zebrafish and are using this model to understand the cellular and molecular changes that lead to defective vascular responses to injury and poor wound healing in aging or diabetes, with an eye toward uncovering new therapeutic targets to promote improved re-vascularization and healing in these contexts.
Vasculature and vascular-associated cells in the meninges
The meninges are an external, enveloping connective tissue that encases the brain, producing cerebrospinal fluid, acting as a cushion against trauma, nourishing the brain via nutrient circulation, and removing waste. Despite its importance, the cell types present in the meninges and their function and embryonic origins are still not well understood. We recently discovered and characterized zebrafish fluorescent granular perithelial cells (FGPs), a novel endothelium-derived perivascular cell population closely associated with meningeal blood vessels, which likely plays a critical role in meningeal function (Figure 3). As discussed further below, we also discovered a bona fide meningeal lymphatic vascular network in the zebrafish. We are currently carrying out additional anatomical and molecular studies to understand the structure and cellular composition of the zebrafish meninges and the function of FGPs, meningeal lymphatics, and other novel meningeal cell populations in health, injury, and disease.
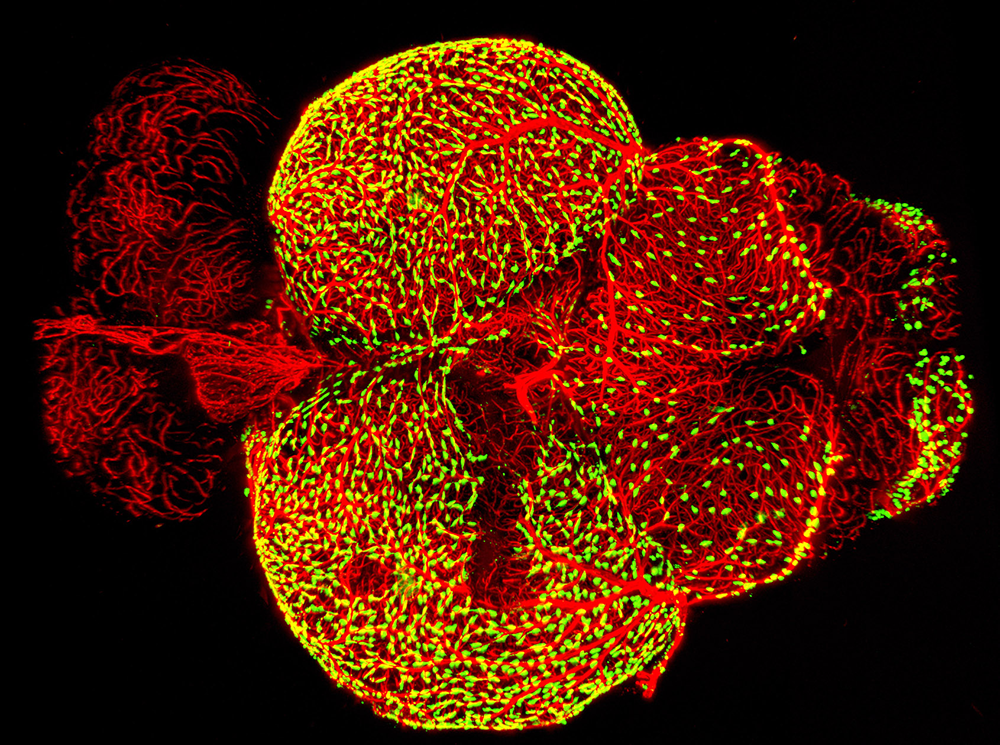
Figure 3. Novel perivascular cells on the zebrafish brain
Confocal micrograph of fluorescent granular perithelial cells (FGPs, green) adhering to the outside of meningeal blood vessels (red) on the brain of a Tg(mrc1a:egfp);Tg(kdrl:cherry) double-transgenic adult zebrafish. We recently showed that FGPs are unique endothelium-derived perivascular cells with unusual scavenging properties that are likely to be critical for brain homeostasis.
Figure 3. Novel perivascular cells on the zebrafish brain
Confocal micrograph of fluorescent granular perithelial cells (FGPs, green) adhering to the outside of meningeal blood vessels (red) on the brain of a Tg(mrc1a:egfp);Tg(kdrl:cherry) double-transgenic adult zebrafish. We recently showed that FGPs are unique endothelium-derived perivascular cells with unusual scavenging properties that are likely to be critical for brain homeostasis.
Specification and patterning of the lymphatic system
The lymphatic system is a vascular system completely separate from the blood circulatory system and comprises an elaborate blind-ended tree of vessels that extensively innervate most of the body, emptying lymph fluid into the venous blood vascular system via several evolutionarily conserved drainage points. The lymphatic system is essential for immune responses, fluid homeostasis, and fat absorption, and is involved in many pathological processes, including tumor metastasis and lymphedema. However, progress in understanding the origins and early development of the system has been hampered by difficulties in observing lymphatic cells in vivo and performing defined genetic and experimental manipulation of the lymphatic system in mammalian model organisms. Our studies demonstrated that zebrafish possess a lymphatic system with many of the morphological, molecular, and functional characteristics of lymphatic vessels found in other vertebrates, providing a powerful model for the purposes of imaging and studying lymphatic development. We have further studied the zebrafish lymphatic system through several projects.
(1) We generated transgenic lines that permit specific visualization, tissue-specific molecular profiling, and functional manipulation of lymphatic vessels, and we are using these transgenic animals to further characterize lymphatic development and function (Figure 4).
Figure 4. Novel lymphatic vascular reporter
Lateral view confocal image of the trunk of a 12 dpf (days post-fertilization) Tg(kdrl:cherry); Tg(mrc1a:egfp) double-transgenic zebrafish with red fluorescent blood vessels and green fluorescent lymphatics. See Jung HM, et al. Development 2017;144:2070 for additional details.
Figure 4. Novel lymphatic vascular reporter
Lateral view confocal image of the trunk of a 12 dpf (days post-fertilization) Tg(kdrl:cherry); Tg(mrc1a:egfp) double-transgenic zebrafish with red fluorescent blood vessels and green fluorescent lymphatics. See Jung HM, et al. Development 2017;144:2070 for additional details.
(2) We characterized and studied novel microRNAs expressed in the lymphatic endothelium and how these small regulatory RNAs influence lymphatic gene expression and lymphatic development.
(3) We discovered a previously unreported lymphatic network in the dural meninges of the zebrafish (Figure 5). Like similar recently discovered meningeal lymphatics surrounding the mammalian brain, the zebrafish meningeal lymphatic network likely plays a critical role in maintaining homeostasis and protecting the brain from mechanical trauma and infection, and we are studying these critical vessels and their role in immune cell interaction and trafficking in homeostasis and following traumatic brain injury (TBI), using new zebrafish cerebrovascular injury models that we recently developed.
Figure 5. Adult zebrafish meningeal lymphatic network
Confocal image of the dorsal head of a Tg(mrc1a:egfp)y251, casper (transparent) adult zebrafish, with mrc1a+FGPs and superficial lymphatics in grey and intracranial meningeal lymphatics pseudo-colored green. Anterior is to the left, right is up.
Figure 5. Adult zebrafish meningeal lymphatic network
Confocal image of the dorsal head of a Tg(mrc1a:egfp)y251, casper (transparent) adult zebrafish, with mrc1a+FGPs and superficial lymphatics in grey and intracranial meningeal lymphatics pseudo-colored green. Anterior is to the left, right is up.
By combining the genetic and experimental tools available in the zebrafish with the ability to perform high-resolution microscopic imaging of vascular structures in living animals, our studies are providing important new insights into the origins and growth of the lymphatic system and molecular mechanisms that are critical both during lymphatic development and in adult lymphatic pathologies.
Epigenetics of development and regeneration
We are using the genetically and experimentally accessible zebrafish and Mexican tetra (Astyanax mexicanus) models to uncover the molecular basis for organ- and tissue-specific epigenetic regulation during development and regeneration through several projects.
Forward-genetic screen for epigenetic regulatory factors
Genetic screens carried out in Drosophila and the nematode Caenorhabditis elegans have been highly successful in identifying genes regulating cell type–specific epigenetic gene regulation in invertebrates, but the molecular mechanisms involved in organ- and tissue-specific epigenetic regulation in vertebrates are still relatively unknown. We developed a novel “EpiTag” zebrafish transgenic reporter line that permits real-time visualization of tissue-specific epigenetic silencing or activation in living animals, making it possible for us to monitor dynamic changes in epigenetic regulation in intact animals during development with cellular resolution. Using the EpiTag transgenic line, we performed the first large-scale F3 genetic screen in a vertebrate to identify recessive mutants in regulators of epigenetic gene silencing or activation (Figure 6). Among other mutants, the screen has yielded epigenetic regulators of liver development, pharynx development, and arterial differentiation, and we are currently pursuing follow-up studies on these mutants.
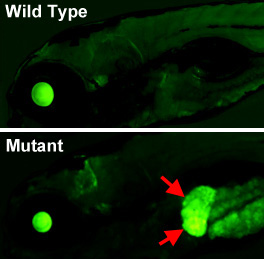
Figure 6. An epigenetic silencing mutant in the zebrafish
Lateral views of the head and anterior trunk of a wild-type (top) and tissue-specific epigenetic silencing mutant (bottom) zebrafish. The mutation causes loss of epigenetic silencing specifically in the liver (red arrows), as visualized with a novel transgenic reporter line developed in our lab, which permits dynamic, tissue-specific visualization of epigenetic silencing in living animals.
Figure 6. An epigenetic silencing mutant in the zebrafish
Lateral views of the head and anterior trunk of a wild-type (top) and tissue-specific epigenetic silencing mutant (bottom) zebrafish. The mutation causes loss of epigenetic silencing specifically in the liver (red arrows), as visualized with a novel transgenic reporter line developed in our lab, which permits dynamic, tissue-specific visualization of epigenetic silencing in living animals.
Epigenetics of regeneration
Epigenetic reprogramming is pivotal for kickstarting dormant developmental programs during regeneration. However, the regulatory mechanisms orchestrating this phenomenon remain elusive. The zebrafish, recognized for its regenerative capabilities, serves as an ideal model to explore this at the molecular level. In addition to serving as a superb tool for studying epigenetic programming occurring during development, and for carrying out genetic screens to identify new epigenetic regulators as described above, the “EpiTag” transgenic line also becomes activated in regenerating tissues and organs. We observe very early and striking activation of the EpiTag reporter in regenerating cells during fin regeneration, with GFP activation serving as a highly specific marker for cells undergoing epigenetic reprogramming at otherwise inaccessible stages of regeneration. We are using EpiTag expression to selectively enrich early-stage regenerating cells using FACS sorting and employing a multi-omics approach including ATAC-seq, bisulfite-seq, bulk RNA-seq, and single-cell RNA-seq to comprehensively profile chromatin accessibility, DNA methylation patterns, gene expression profiles, and single-cell transcriptomes in regenerating cells. This approach has led to the identification of novel epigenetic regulatory genes, molecular complexes, and pathways that we are currently further studying.
Epigenetics of vascular adaptations in cavefish
In addition to eye and pigment loss and other adaptations, Astyanax cavefish (Figure 7) have unusual vascular and metabolic adaptations that allow them to survive hypoxic conditions, chronic and long-term food deprivation, and cold. As we recently showed for loss of eyes, changes in epigenetic regulation may underlie the rapid evolution of cavefish vascular and metabolic adaptations. We are generating new transgenic tools that will allow us to use imaging and transcriptional and epigenetic profiling to investigate differences in vascular endothelial cells in cavefish compared with surface morphs of Astyanax. We will follow up on these findings to elucidate how differential epigenetic signatures influence divergent vascular endothelial behavior in surface and cave morphs. We are focusing in particular on the gills, a vascular-rich gas-exchange tissue analogous to lungs that shows adaptive changes to hypoxic cave environments, which we are studying to help understand the pathologic adaptations of lung endothelial cell to hypoxia in human lung diseases.
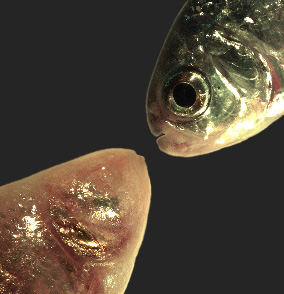
Figure 7. Mexican tetra cave- and surface fish
The Mexican tetra Astyanax mexicanus is a freshwater fish native to parts of southern Texas and eastern and central Mexico, which exists in both surface-dwelling (‘surface morphs,’ top right) and very closely related cave-dwelling (‘cave morphs,’ bottom left) populations. Cave morphs have a series of uniquely evolved adaptations, including loss of eyes and pigment, dramatically altered metabolism, altered vascular function, and altered sleep regulation and behavior. Results from our lab suggest that altered DNA methylation and resulting coordinated changes in the expression of large sets of genes have helped drive at least some of this rapid evolutionary change.
Figure 7. Mexican tetra cave- and surface fish
The Mexican tetra Astyanax mexicanus is a freshwater fish native to parts of southern Texas and eastern and central Mexico, which exists in both surface-dwelling (‘surface morphs,’ top right) and very closely related cave-dwelling (‘cave morphs,’ bottom left) populations. Cave morphs have a series of uniquely evolved adaptations, including loss of eyes and pigment, dramatically altered metabolism, altered vascular function, and altered sleep regulation and behavior. Results from our lab suggest that altered DNA methylation and resulting coordinated changes in the expression of large sets of genes have helped drive at least some of this rapid evolutionary change.
Additional Funding
- K99/R00 Award (to Leah Greenspan)
- Japan Society for the Promotion of Science (JSPS) Award (to K. Taimatsu)
- Japan Society for the Promotion of Science (JSPS) Award (to K. Inoue)
- PRAT Fellowship (to Jong Park)
- NICHD Intramural Research Fellowship (to Aurora Kraus)
- NICHD Early Career Award (to Miranda Marvel)
- NICHD Early Career Award (to Leah Greenspan)
- NICHD Early Career Award (to Kiyohito Taimatsu)
Publications
- The axillary lymphoid organ - an external, experimentally accessible immune organ in the zebrafish. bioRxiv 2024 2024.07.25.605139
- Live imaging of cutaneous wound healing in zebrafish. J Invest Dermatol 2024 144:888–897
- Dermal Dive: an overview of cutaneous wounding techniques in zebrafish. J Invest Dermatol 2024 144:1430–1439
- Angiogenesis is limited by LIC1‐mediated lysosomal trafficking. Angiogenesis 2024 27:943–962
- Development and structure of the lymphoid system. In: Clinical Immuno-Oncology. J. E. Niederbuber, Ed. Elsevier Press, New York 2022 95–103
- LSD1 promotes the egress of hematopoietic stem and progenitor cells into the bloodstream during the endothelial-to-hematopoietic transition. Dev Biol 2023 501:92–103
Collaborators
- Andreas Baxevanis, PhD, Computational and Statistical Genomics Branch, NHGRI, Bethesda, MD
- Harold Burgess, PhD, Section on Behavioral Neurogenetics, NICHD, Bethesda, MD
- Ryan Dale, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
- Louis Dye, Microscopy and Imaging Core, NICHD, Bethesda, MD
- Kendrick L. Highsmith, Division of Developmental Biology, NIAID, Bethesda, MD
- James Iben, PhD, Molecular Genomics Laboratory, NICHD, Bethesda, MD
- Sumio Isogai, PhD, Iwate Medical University, Morioka, Japan
- William R. Jeffery, PhD, University of Maryland, College Park, MD
- Paul Liu, MD, PhD, Genetics and Molecular Biology Branch, NHGRI, Bethesda, MD
- Richard Maraia, MD, Section on Molecular and Cell Biology, NICHD, Bethesda, MD
- Gennady Margolin, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
- Yoh-suke Mukouyama, PhD, Laboratory of Stem Cell and Neuro-Vascular Biology, NHLBI, Bethesda, MD
- Lisa M. Price, PhD, Division of Developmental Biology, NICHD, Bethesda, MD
- Radu V. Stan, MD, PhD, Geisel School of Medicine at Dartmouth, Lebanon, NH
Contact
For more information, email weinsteb@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/weinstein.