Physiology, Psychology, and Genetics of Obesity
- Jack A. Yanovski,
MD, PhD, Head, Section on Growth and Obesity - Sheila M. Brady, RN, FNP, Nurse Practitioner
- Tusharkumar Patel, PhD, Visiting Fellow
- Diana Elizondo, PhD, Postdoctoral Fellow
- Robin Roberson, MS, Technician
- Nasreen Moursi, BA, Graduate Student, Guest Researcher
- Megan Parker, MS, Graduate Student, Guest Researcher
- Aditi Venkatesh, BA, Graduate Student, Guest Researcher
- Praise Adekola, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Sarah J. Baker, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Diana Benitez-Alvarado, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Bess Bloomer, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Jocelyn Chen, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Benjamin Cole, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Melanie Hollis, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Eliza Jansujwicz, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Jeremiah Jones, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Julia Lazareva, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Alexa P. Lyman, BS, Postbaccalaureate Intramural Research Training Award Fellow
- A. Otiti Mayo, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Ejike Nwosu, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Jennifer Te-Vazquez, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Bao-Vi Tran, BS, Postbaccalaureate Intramural Research Training Award Fellow

The prevalence of overweight and obesity in children and adults has tripled during the past 40 years, an alarming rise in body weight that has likely occurred because the current environment affords easy access to energy-dense foods and requires less voluntary energy expenditure. However, such an environment leads to obesity only in those individuals whose body weight–regulatory systems are not able to control body adiposity with sufficient precision in our high-calorie/low-activity environment, which suggests that there are subgroups in the U.S. with a uniquely high susceptibility to weight gain under the prevailing environmental conditions. Our primary goal is to elucidate the genetic underpinnings of the metabolic and behavioral endo-phenotypes that contribute to the development of obesity in children. Using our unique longitudinal cohorts of children who have undergone intensive metabolic and behavioral phenotyping, we examine genetic and phenotypic factors predictive of progression to adult obesity in children who have obesity or are in the “pre-obesity” state, permitting characterization of phenotypes unconfounded by the impact of obesity itself. Once they are identified as linked to obesity, we can study genetic variants that impair gene function intensively. We expect that these approaches will improve our ability to predict which children are at greatest risk for obesity and its comorbid conditions and will lead to more targeted, etiology-based prevention and treatment strategies for pediatric obesity, some of which have been studied by our research group.
Genetic factors important for childhood body-weight regulation
We are conducting studies to understand how the melanocortin 3 receptor (MC3R) affects energy regulation. We previously found an MC3R haplotype that is associated with adiposity in children and adults. Children and adults who were homozygous variant for both C17A and G241A polymorphisms had significantly greater fat mass and higher plasma levels of insulin and leptin than unaffected or heterozygous children and ate more at laboratory test meals. In vitro studies subsequently found that signal transduction and protein expression were significantly lower for the double mutant MC3R. The transgenic ‘knock-in’ mice we made to replace murine Mc3r with wild-type human (MC3RhWT/hWT) and double-mutant (C17A+G241A) human (MC3RhDM/hDM) MC3R indicated that MC3RhDM/hDM mice had greater weight and fat mass but reduced length and fat-free mass than MC3RhWT/hWT. Interestingly, MC3RhDM/hDM mice have greater feeding efficiency than MC3RhWT/hWT. MC3RhDM/hDM bone– and adipose tissue–derived mesenchymal stem cells (MSCs) differentiate into adipocytes that accumulate more triglyceride than MC3RhWT/hWT MSCs. MC3RhDM/hDM, thus impacting nutrient partitioning to generate increased adipose tissue. Our studies this year found that at least part of the reason for adipose tissue hypertrophy in MC3RhDM/hDM mice is that they have impaired lipolysis, with reduced hormone-sensitive lipase (HSL) phosphorylation in response to beta-adrenergic stimulation (Figure 1). We also have substantial data indicating a novel role for the MC3R in the regulation of hepatic autophagy (Figure 2). Both full knockouts and MC3RhDM/hDM mice show impaired autophagy flux, while specific ligands for MC3R can activate hepatic autophagy pathways. Using tissue-specific knockout- and reactivation models, we now plan to study the importance of leukocyte MC3R hepatic and adipose tissue MC3R for whole body homeostasis.
Figure 1. Studies of lipolysis in a human MC3R variant
The MC3RhDM/hDM variant is associated with pediatric-onset obesity. In mice whose MC3R was replaced by human versions of the gene, we found that adipocytes from MC3RhDM/hDM mice had (A) lower lipolysis than mice with the human common (wild-type) version of the human gene (MC3RhWT/hWT); we found the same reduced lipolysis (B) in people with the MC3RhDM/hDM variant. Compared with (C) MC3RhWT/hWT mice, mice with MC3RhDM/hDM (D) had much lower ability to phosphorylate a key enzyme for lipolysis, and did not associate HSL (red) with lipid droplets (green), so that much less yellow (co-localized) signal was found in MC3RhDM/hDM mice.
Figure 1. Studies of lipolysis in a human MC3R variant
The MC3RhDM/hDM variant is associated with pediatric-onset obesity. In mice whose MC3R was replaced by human versions of the gene, we found that adipocytes from MC3RhDM/hDM mice had (A) lower lipolysis than mice with the human common (wild-type) version of the human gene (MC3RhWT/hWT); we found the same reduced lipolysis (B) in people with the MC3RhDM/hDM variant. Compared with (C) MC3RhWT/hWT mice, mice with MC3RhDM/hDM (D) had much lower ability to phosphorylate a key enzyme for lipolysis, and did not associate HSL (red) with lipid droplets (green), so that much less yellow (co-localized) signal was found in MC3RhDM/hDM mice.
Figure 2. Hepatic recovery of Mc3r demonstrates the role of Mc3r in autophagy.
Mice with recovery of Mc3r only in the liver (Mc3rTB/TB Alb-Cre++, blue bars and circles) have improved body weight (A, B), fat mass (C, D), liver triglycerides (TG) (E), and hepatic autophagy (F) compared with complete Mc3r inactivation (Mc3rTB/TB, black bars and circles). There is almost complete restoration of normal hepatic TG and autophagy markers compared with wild-type mice (BL/6, open bars and circles).
Figure 2. Hepatic recovery of Mc3r demonstrates the role of Mc3r in autophagy.
Mice with recovery of Mc3r only in the liver (Mc3rTB/TB Alb-Cre++, blue bars and circles) have improved body weight (A, B), fat mass (C, D), liver triglycerides (TG) (E), and hepatic autophagy (F) compared with complete Mc3r inactivation (Mc3rTB/TB, black bars and circles). There is almost complete restoration of normal hepatic TG and autophagy markers compared with wild-type mice (BL/6, open bars and circles).
Physiology, metabolism, and psychology of childhood body-weight regulation
Our studies are directed at understanding the physiological, psychological, and metabolic factors that place children at risk for undue weight gain. As part of these studies, we examined how best to measure eating-related psychopathology, insulin sensitivity, changes in body composition, energy intake, and energy expenditure in children, and we studied the short- and long-term stability of the components of the metabolic syndrome. We previously found that leptin is an important predictor of weight gain in children, and we identified children with hyperleptinemia and proximal leptin-signaling pathway mutations. We also found hyperleptinemia to be out of proportion with body fat mass in children with psychological loss of control (LOC) over-eating. Such data suggest the importance of leptin resistance as a factor stimulating weight gain, and they led to recent explorations of other syndromes associated with obesity that may cause dysregulation of leptin signaling, including WAGR, Bardet-Biedl, Alström, and Smith-Magenis syndromes [Reference 1]. Current studies are directed at understanding additional genetic, physiological, and psychological factors that place children at risk for undue weight gain, including humoral factors, sleep, negative affective states such as emotional dysregulation, food reinforcement and food-related inhibitory control, attention biases towards high palatability foods, alexithymia, executive functioning, and LOC eating. Some recent initiatives have targeted insulin resistance in girls at high risk for type 2 diabetes because of obesity and a family history of diabetes.
Our evaluations concentrating on binge-eating behaviors in children suggest that such behaviors are also associated with adiposity in children and abnormalities in metabolism. We found that certain eating patterns may predict future weight gain in children at risk for obesity: children reporting binge-eating behaviors, such as loss of control over eating, gained, on average, an additional 2.4 kg of weight per year compared with non-binge-eating children. Our data also suggest that children endorsing binge eating consume more energy during meals. Actual intake during buffet meals averaged 400 kcal more in children with binge eating, but despite their greater intake, such children reported shorter-lived satiety than children without binge-eating episodes. The ability to consume large quantities of palatable foods, especially when coupled with lower subsequent satiety, may play a role in the greater weight gain found in binge-eating children. We demonstrated, among cohorts of lean and obese youth, that youth with LOC eating had higher serum leptin and are at significantly greater risk for worsening of components of the metabolic syndrome than those without LOC episodes, even after adjusting for adiposity and other relevant covariates. These data suggest that interventions targeting disordered eating behaviors may be useful in preventing excessive fat gain in children prone to obesity and have led to trials of preventative strategies related to binge eating. We previously found that binge-eating behaviors were linked to attentional biases for high-palatability foods. In 2024, we reported results of a pilot attention-retraining study using smartphones to deliver the intervention in the natural environment, which showed that, using magnetoencephalography to interrogate attention-relevant brain areas, the attention-retraining (AR) group had reduced initial engagement in brain areas associated with reward response and subsequent increased goal-directed attention and action control [Reference 2].
We study normal-weight children and adolescents, children who already have obesity, and the children of parents with obesity who do not have obesity, in order to determine the factors that are most important for the development of the complications of obesity in youth. We also examine body composition, leptin concentration, metabolic rate, insulin sensitivity, glucose disposal, energy intake at buffet meals, energy expenditure, and genetic factors believed to regulate metabolic rate and body composition. Psychological and behavioral factors, such as propensity to engage in binge-eating behavior (Figure 3), and sleep are also studied. Children are being followed longitudinally into adulthood. In our protocols, we study actual food consumption of children during meals, to elucidate differences in the calorie and macronutrient content of meals and the circulating hormones related to hunger and satiety. We found that eating in the absence of physiological hunger is a replicable trait that appears linked to obesity. We also investigated the role of sedentary behaviors, finding that glucose homeostasis was markedly improved in children with overweight or obesity who engaged in moderate activity for just three minutes every half hour, versus remaining sedentary.
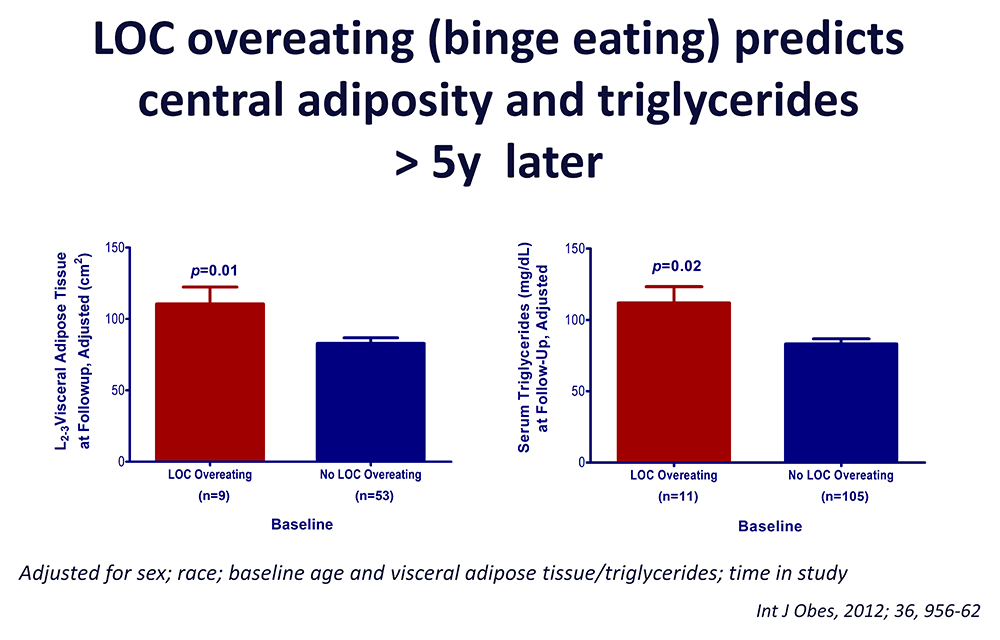
Figure 3. Loss of control (LOC) eating and metabolic complications in a longitudinal study
On average (±SE), children who engaged in binge eating at baseline had more visceral adipose tissue at the L2-3 intervertebral space at follow-up than children who did not engage in binge eating at baseline, adjusting for sex, race, baseline age, baseline visceral adipose tissue at L2-3, and time in study (P = 0.01). On average (±SE), children who engaged in binge eating at baseline had higher follow-up triglycerides than children who did not engage in binge eating at baseline, adjusting for sex, race, baseline age, body mass index (kg/m2), baseline triglycerides, and time in study (P = 0.02).
Figure 3. Loss of control (LOC) eating and metabolic complications in a longitudinal study
On average (±SE), children who engaged in binge eating at baseline had more visceral adipose tissue at the L2-3 intervertebral space at follow-up than children who did not engage in binge eating at baseline, adjusting for sex, race, baseline age, baseline visceral adipose tissue at L2-3, and time in study (P = 0.01). On average (±SE), children who engaged in binge eating at baseline had higher follow-up triglycerides than children who did not engage in binge eating at baseline, adjusting for sex, race, baseline age, body mass index (kg/m2), baseline triglycerides, and time in study (P = 0.02).
Treatment of obesity and the co-morbid conditions associated with obesity
Given the rapid increase in the prevalence of obesity, the development of treatments for obesity in children and adults is urgently needed, and current pharmacologic approaches show marked efficacy in adults [Reference 4], but remain more limited in children. In several clinical protocols, we examined approaches for the prevention and treatment of excessive body weight. In 2024, we reported the efficacy of the glucagon-like peptide 1 agonist liraglutide in adolescents who continue to have obesity despite sleeve gastrectomy bariatric surgery, finding that the body mass index (BMI) decreased by 4.3% with liraglutide. Adolescents who had poor initial response to their surgical procedure (less than 20% BMI reduction at BMI nadir) had less weight loss with liraglutide. Fasting glucose and hemoglobin A1C concentrations fell significantly. No serious treatment-emergent adverse events were reported. We studied the effects of modulation of the leptin signaling pathway with the melanocortin agonist setmelanotide in patients with proximal signaling defects in patients with Bardet Biedl syndrome and Smith Magenis syndrome [Reference 1]. Most recently, we completed another study of specific pharmacotherapy for patients with Prader-Willi syndrome using diazoxide choline extended release (DCCR), first during a placebo-controlled period and then versus a contemporaneously collected untreated group [Reference 4]. DCCR administration resulted in significant improvements in hyperphagia scores and even greater improvements in those with more severe baseline hyperphagia. We saw significant improvements in aggression, anxiety, and compulsivity, and reductions in leptin, insulin, and insulin resistance, as well as a significant increase in adiponectin and lean body mass; disease severity was significantly reduced, as assessed by clinician and caregiver. These latest trials are examples of precision-medicine approaches to treat obesity.
We also completed a novel randomized-controlled pilot trial of colchicine to ameliorate the inflammation associated with obesity and thus improve its complications. Adults with obesity and metabolic syndrome, but who did not have diabetes, were randomized to 0.6 mg colchicine or placebo capsules twice daily for three months. Compared with placebo, colchicine significantly reduced C-reactive protein and erythrocyte sedimentation rate (Figure 4). The significant changes in homeostatic-model assessment of insulin resistance (HOMA-IR), fasting insulin, and glucose effectiveness suggested metabolic improvements in the colchicine versus placebo group, although we found few differences in the stool microbiome of colchicine-treated participants [Reference 5]. We are now conducting a larger, adequately powered study to determine whether colchicine improves insulin resistance and other measures of metabolic health in at-risk individuals.
Figure 4. Effects of colchicine on inflammatory and metabolic measures
Metabolic and inflammatory changes after three months of study medication in participants randomized to colchicine (N=21) or placebo (N=19).
A. Insulin sensitivity (SI).
B. Fasting glucose.
C. Fasting insulin.
D. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR).
E. High sensitivity C-reactive protein (hsCRP).
F. Erythrocyte sedimentation rate (ESR).
G. White blood cell count (WBC).
H. Neutrophil count.
I. Platelet count. Data are presented as mean ± SEM.
Figure 4. Effects of colchicine on inflammatory and metabolic measures
Metabolic and inflammatory changes after three months of study medication in participants randomized to colchicine (N=21) or placebo (N=19).
A. Insulin sensitivity (SI).
B. Fasting glucose.
C. Fasting insulin.
D. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR).
E. High sensitivity C-reactive protein (hsCRP).
F. Erythrocyte sedimentation rate (ESR).
G. White blood cell count (WBC).
H. Neutrophil count.
I. Platelet count. Data are presented as mean ± SEM.
Additional Funding
- Rhythm Pharmaceuticals, Inc.: Setmelanotide (RM-493; Rhythm Pharmaceuticals, Inc.) phase 2 open-label treatment trials in patients with rare genetic disorders of obesity. 2017–2024.
- Soleno Therapeutics, Inc. Grant support to fund an RCT testing diazoxide choline sustained release tablets in patients with Prader-Willi syndrome and hyperphagia. 2018–2025.
- 5 K99 DK136921. Funds postdoctoral fellowship for Diana Elizondo, PhD, 2023–2025
Publications
- Investigation of setmelanotide, an MC4R agonist, for obesity in individuals with Smith-Magenis syndrome. Obes Res Clin Pract 2024 18(4):301–307
- A pilot randomized control trial testing a smartphone-delivered food attention retraining program in adolescent girls with overweight or obesity. Nutrients 2024 16(20):3456
- Approach to obesity treatment in primary care: a review. JAMA Intern Med 2024 184(7):818–29
- Diazoxide choline extended-release tablet in people with Prader-Willi syndrome: results from long-term open-label study. Obesity (Silver Spring) 2024 32(2):252–61
- Colchicine effects on the gut microbiome in adults with metabolic syndrome. Biosci Microbiota Food Health 2023 42(4):236–42
Collaborators
- Sean Agbor-Enoh, MD, PhD, Applied Precision Omics, NHLBI, Bethesda, MD
- Britni Belcher, PhD, MPH, Keck School of Medicine, University of Southern California, Los Angeles, CA
- Karen Berman, PhD, Section on Integrative Neuroimaging, NIMH, Bethesda, MD
- Kong Chen, PhD, Clinical Endocrinology Branch, NIDDK, Bethesda, MD
- Bobby Cheon, PhD, Social and Behavioral Sciences Branch, NICHD, Bethesda, MD
- Ross Crosby, PhD, Director of Biomedical Statistics, Neuropsychiatric Research Institute, Fargo, ND
- Pradeep Dagur, PhD, Flow Cytometry Core, NHLBI, Bethesda, MD
- Andrew P. Demidowich, MD, Johns Hopkins University School of Medicine, Baltimore, MD
- Joel Elmquist, DVM, PhD, UT Southwestern Medical Center, Dallas, TX
- Scott Engel, PhD, University of North Dakota School of Medicine and Health Sciences, Grand Forks, ND
- Oksana Gavrilova, PhD, Mouse Metabolism Core Laboratory, NIDDK, Bethesda, MD
- Robert Haws, MD, Marshfield Clinic Research Institute, Marshfield, WI
- Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Baton Rouge, LA
- Joel Hirschhorn, MD, PhD, Boston Children's Hospital, Boston, MA
- Sergey Leikin, PhD, Section on Physical Biochemistry, NICHD, Bethesda, MD
- Nazrat Mirza, MD, Childrens National Medical Center, Washington, DC
- Cara Olsen, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Vipul Periwal, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Daniel Pine, MD, Section on Development and Affective Neuroscience, NIMH, Bethesda, MD
- Alan Remaley, MD, PhD, Lipoprotein Metabolism Laboratory, NIH Clinical Center, Bethesda, MD
- Peter J. Schmidt, MD, Section on Behavioral Endocrinology, NIMH, Bethesda, MD
- Dale A. Schoeller, PhD, University of Wisconsin, Madison, WI
- Lauren B. Shomaker, PhD, University of Colorado, Boulder, CO
- Ninet Sinaii, PhD, Biostatistics and Clinical Epidemiology Service, NIH Clinical Center, Bethesda, MD
- Ann C. Smith, MA, DSc (hon), Medical Genetics Branch, NHGRI, Bethesda, MD
- John Speakman, PhD, University of Aberdeen, Aberdeen, United Kingdom
- Marian Tanofsky-Kraff, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Martin Wabitsch, MD, PhD, Universitätsklinikum Ulm, Ulm, Germany
- Andrew Waters, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Denise E. Wilfley, PhD, Washington University School of Medicine, St. Louis, MO
- Joshua Zimmerberg, MD, PhD, Section on Integrative Biophysics, NICHD, Bethesda, MD
Contact
For more information, email yanovskj@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/yanovski.