You are here: Home > Section on Cellular and Synaptic Physiology
Hippocampal Interneurons and Their Role in the Control of Network Excitability

- Chris J. McBain, PhD, Head, Section on Cellular and Synaptic Physiology
- Christian Cea del Rio, BS, Graduate Student
- Michael Daw, PhD, Visiting Fellow
- Brian Erkkila, PhD, Visiting Fellow
- Tsz-wan Michelle Ho, BS, Graduate Student
- Brian Jefferies, BS, Biologist
- Elizabeth Mayne, BSc, Graduate Student
- Kenneth Pelkey, PhD, Staff Scientist
- Madhav Sukumaran, BSc, Graduate Student
- Christine Torborg, PhD, Visiting Fellow
- Ludovic Tricoire, PhD, Visiting Fellow
- Xiaoqing Yuan, MSc, Biologist
GABAergic inhibitory interneurons constitute a population of hippocampal neurons whose high degree of anatomical and functional divergence make them suitable candidates for controlling the activity of large populations of principal neurons. Interneurons (INT) receive strong excitatory glutamatergic innervation via numerous anatomically distinct afferent projections; recent evidence has demonstrated that the molecular composition of the AMPA-preferring class of glutamate receptors expressed at INT synapses is often distinct from that of receptors found at principal cell synapses. Our goal is to identify the types of ligand-gated ion- and voltage-gated ion channels and the roles they play in controlling the cortical network. Specifically, we strive to understand the functions of these receptors in regulating synaptic and neuronal excitability, short- and long-term plasticity, and the development and pathophysiology of the mammalian cortical structure. Through this research, we envision a better understanding of the specific mechanisms regulating excitability in precise cohorts of central nervous system cells and thus more targeted and valid methods of treatment of a wide array of central nervous system disease states.
State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit
At hippocampal mossy fiber– (MF) st. lucidum interneuron (SLIN) synapses, mGluR7 serves as a metaplastic switch controlling bidirectional plasticity. During high-frequency stimulation (HFS), mGluR7 activation triggers presynaptic long-term depression (LTD) due to persistent P/Q-type Ca2+ channel inhibition. However, following mGluR7 internalization, HFS produces presynaptic LTP. Surprisingly, LTP does not simply reflect molecular reversal of Ca2+ channel depression. Rather mGluR7 activation/internalization controls plasticity polarity by gating cAMP sensitivity of release. While naive, surface mGluR7–expressing MF–SLIN synapses are insensitive to cAMP elevation, synapses that have internalized mGluR7 potentiate robustly, following cAMP increases. Moreover, MF–SLIN LTP requires adenylate cyclase (AC) and protein kinase A (PKA) activities. We also discovered an association between mGluR7 and RIM1α, an active zone molecule required for AC/PKA–dependent presynaptic LTP. Importantly, the mGluR7–RIM1α interaction is regulated by mGluR7 activation, and mice lacking RIM1α are deficient in MF–SLIN LTP. We conclude that state-dependent cAMP sensitivity controlled by mGluR7–RIM1α interactions underlies MF–SLIN metaplasticity.
Burst firing induces postsynaptic depolarization-induced LTD (DiLTD) at developing mossy fiber–CA3 pyramid synapses
Mossy fiber–CA3 pyramid (MF–PYR) synapses develop postnatally, providing a tractable model of synapse maturation in a defined circuit. Hippocampal CA3 pyramidal neurons (PYRs) exhibit intense burst firing (BF) early in development, concomitant with the period of mossy fiber (MF) development. However, whether developing MF–PYR synapses utilize PYR BF to promote MF synapse maturation remains unknown. Recently, we demonstrated that transient tonic depolarization of postsynaptic PYRs induces a persistent postsynaptic form of LTD (depolarization-induced LTD, DiLTD) at immature MF–PYR synapses. DiLTD induction is NMDAR–independent but does require postsynaptic Ca2+ influx through L-type voltage-gated Ca2+ channels (LVGCCs), and is expressed as a reduction in AMPAR function through the loss of GluR2–lacking AMPARs present at immature MF–PYR synapses. We examined whether more physiologically relevant phasic L-VGCC activation by PYR action potential (AP) BF activity patterns can trigger DiLTD. Using combined electrophysiological and Ca2+ imaging approaches, we demonstrate that PYR BF effectively drives L-VGCC activation and that brief periods of repetitive PYR BF, produced by direct current injection or intrinsic network activity, induces NMDAR–independent LTD by promoting Ca2+ influx through the activated L-VGCCs. This BF–induced LTD, just like DiLTD, is specific for developing MF–PYR synapses, is PICK1 dependent, and is expressed postsynaptically. Our results demonstrate that DiLTD can be induced by phasic L-VGCC activation driven by PYR BF, suggesting the engagement of natural PYR network activity patterns for MF synapse maturation.
The balance of excitatory and inhibitory synaptic currents is maintained during short-term plasticity in the CA3 hippocampus
Within the hippocampus, glutamatergic MF synapses onto CA3 pyramidal cells (PCs) exhibit strong frequency-dependent facilitation. However, previous studies suggested that polysynaptic inhibitory networks within CA3 depress during repetitive stimulation, which would make the circuit vulnerable to pathological hyperexcitability. We demonstrated that polysynaptic MF–driven IPSCs within CA3 facilitate. In fact, polysynaptic inhibitory postsynaptic current (IPSC) facilitation matches MF–driven excitatory postsynaptic current (EPSC) facilitation, resulting in a constant EPSC–IPSC ratio throughout the course of the train. Given that the IPSC was delayed relative to the EPSC, the peak amplitude of the EPSC before the onset of inhibition progressively increased during the train. Within the feedforward inhibitory network, MF–inhibitory interneuron (INT) EPSCs can either facilitate or depress; however, monosynaptic INT–CA3 PC IPSCs never facilitate. DICE-K transgenic mice, which do not have feedback inhibition, also exhibit facilitating polysynaptic IPSCs. Thus, the feedforward inhibitory network generates facilitating polysynaptic IPSCs to balance the facilitation of MF–CA3 PC EPSCs, maintaining network stability while increasing AP probability.
Asynchronous transmitter release from CCK-containing inhibitory interneurons is widespread and target-cell independent
Chemical synaptic transmission classically occurs when neurotransmitter is released from a presynaptic terminal in response to an AP in a tightly time-locked fashion. Neurotransmitter release at most central synapses is synchronized to the timing of presynaptic action potentials. However, it has been shown at several central synapses that, in addition to synchronous release, some transmitter may be released in a longer and less precise time window such that release is asynchronous and not locked to individual presynaptic triggers. We showed that three classes of DSI- (depolarization-induced suppression of inhibition) expressing, presumed cholecystokinin- (CCK) containing, hippocampal interneurons show highly asynchronous release in response to trains of action potentials. This asynchrony is correlated with the class of presynaptic interneuron but is unrelated to their postsynaptic cell target. Asynchronous (from CCK–containing interneurons) and synchronous- (from parvalbumin-containing interneurons) release show a near identical calcium dependence, suggesting a divergence of release mechanisms downstream of the calcium sensor. Asynchronous IPSCs include very large (up to 500 pA/7nS) amplitude events, which persist in low extracellular calcium and strontium, showing that the events result from quantal transmitter release at single release sites. The fact that asynchronous release from CCK–containing interneurons is a widespread phenomenon indicates a fundamental role for these cells within the hippocampal network that is distinct from the phasic inhibition provided by PV-containing interneurons.
Hippocampal INT subtypes are associated with distinct muscarinic receptor mRNA expression profiles
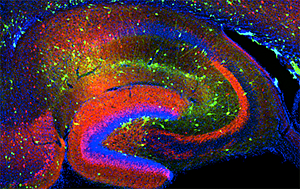
Click image to enlarge.
Figure 1. Interneurons in the GAD-65 GFP mouse
Numerous subpopulations are present within a hippocampal section derived from a GAD-65 GFP transgenic mouse.
INTs in neocortex and hippocampus differentially undergo muscarinic receptor (mAChR) activation, but it is not clear whether muscarinic phenotypes of INTs arise from differences in the expression or localization of mAChRs, intrinsic voltage-gated ion channels, calcium-buffering capacity, or some combination of these factors. Using single-cell PCR, biocytin labeling, and transgenic mice expressing GFP in PV (line B13) or GAD65-expressing INTs (Figure 1), we determined mAChR mRNA expression profiles (M1R–M5R) in morphologically, electrophysiologically, and neurochemically identified INT populations. We based neurochemical profiles for each INT on nine neurochemical markers: GAD67, GAD65, calretinin, PV, calbindin (CB), somatostatin (SOM), neuropeptide Y (NPY), vasoactive intestinal peptide (VIP), and CCK. In all CA1 PV-GFP cells identified as fast-spiking basket cells (BC), we detected PV mRNA but not CB, CCK, VIP, or SOM mRNA. PV+ BCs expressed M1 (100%), M2 (40%), M3 (20%), and M4 (60%) muscarinic receptor mRNA. In contrast, CA1 GAD65 GFP+ INTs expressed abundant CCK (92%), and CB (69%) mRNA, as did non–fast-spiking Schaeffer collateral–associated and CCK BC populations. We probed a subset of GAD65 GFP+ cells for mAChR mRNAs. Similar to PV+ BCs, M1 mRNA was present in all GAD65 GFP+ cells. In contrast, M2 mRNA was absent while M3 mRNA was expressed in higher relative abundance (71%) than in PV-GFP+ cells. The data are consistent with M1 antagonist sensitivity to mAChR responses in PV-GFP and GAD65 cell types. Immunocytochemical evidence for M2Rs on PV+ boutons prompted us to examine the functional consequence of M2Rs on PV-GFP INTs. In PV+ BC–pyramidal cell pairs, bath application of the muscarinic receptor agonist muscarine reduced the unitary IPSC amplitude in wild-type, but not in PV-GFPxM2 KO, mice. Thus, our data provide evidence not only for the differential expression of mAChR subtypes between INT subtypes but also for differential targeting within an INT subtype.
Neurochemical identity governs cholinergic phenotype across hippocampal BC networks
Synaptic release of acetylcholine into the hippocampus induces oscillations associated with behavioral learning. Although these oscillations are sustained through the participation of principal cells and distinct INT subtypes, the organizing principles governing cholinergic neuromodulation of hippocampal INTs remain unknown. To investigate whether cholinergic neuromodulation depends on neurochemical identity, we examined muscarinic acetylcholine receptor (mAChR) activation of two perisomatically targeting interneuron subtypes, PV and CCK BCs. Activation of mAChRs on CCK BCs was accompanied by M3 mAChR–dependent changes in firing frequency and spike accommodation, but the emergence of an afterdepolarization was M1/M3–dependent. In contrast, PV BCs, lacking M3 mAChRs, exhibited modest M1 mAChR–induced increases in excitability. We found that mAChR activation of M3 mAChR–lacking CCK BCs transformed CCK BCs to the mAChR response profile of PV BCs. Therefore, cholinergic modulation of hippocampal targets is orchestrated through cell type– and mAChR subtype–specific conductances present on neurochemically distinct BC subtypes.
Common origins of hippocampal Ivy and nitric oxide synthase–expressing neurogliaform cells
The hippocampus widely expresses the neuronal isoform of nitric oxide synthase (nNOS), an enzyme frequently found in neuropeptide Y– (NPY) expressing GABAergic INTs. So far, we have identified two distinct cell types in the mammalian cortex that are positive for nNOS/NPY: the Ivy cell (IvC) and neurogliaform cell (NGC). While IvCs are located mainly in the stratum oriens and stratum radiatum, NGCs are typically found in the stratum lacunosum moleculare. Both cell types have a dense axonal arbor and are involved in slow synaptic transmission. The axons of NGCs overlap with the glutamatergic input from the entorhinal cortex while the axonal fields of IvCs are aligned with the CA3 input to stratum oriens and stratum radiatum. GABAergic INTs critically regulate cortical computation through exquisite spatiotemporal control over excitatory networks. Although nNOS+ INTs constitute the largest hippocampal interneuron cohort, their origin and specification remain unknown. Thus, given that NGCs and IvCs represent the main nNOS+ interneurons, we investigated their developmental origins. Although considered distinct interneuron subtypes, NGCs and IvCs exhibited similar neurochemical and electrophysiological signatures, including NPY expression and late spiking. Moreover, lineage analyses, including loss-of-function experiments and inducible fate mapping, indicated that nNOS+ IvCs and NGCs are both derived from medial ganglionic eminence (MGE) progenitors under control of the transcription factor Nkx2-1. Surprisingly, a subset of NGCs lacking nNOS arises from caudal ganglionic eminence (CGE) progenitors. Thus, while MGE-derived nNOS+ NGCs and IvCs appear identical by every parameter examined, a CGE origin distinguishes a discrete population of nNOS− NGCs.
Additional Funding
- Dr. Torborg was the recipient of a PRAT Fellowship.
Publications
- Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ, Roche KW. Co-requirements of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron 2008 58:736-748.
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from CCK-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci 2009 29:11112-111122.
- Pelkey KA, Topolnik L, Yuan X-Q, Lacaille J-C, McBain CJ. State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit. Neuron 2008 60:980-987.
- Ho TW, Pelkey KA, Pelletier JG, Huganir RL, Lacaille J-C, McBain CJ. Burst firing induces postsynaptic LTD at developing mossy fiber-CA3 pyramid synapses. J Physiol 2009 587:4441-4454.
- McBain CJ, Kauer JA. Presynaptic plasticity: targeted control of inhibitory networks. Curr Opin Neurobiol 2009 19:1-9.
Collaborators
- Bruno Cauli, PhD, Université Pierre et Marie Curie, Paris, France
- Gordon Fishell, PhD, New York University, New York, NY
- Richard Huganir, PhD, Howard Hughes Medical Institute, The Johns Hopkins University, Baltimore, MD
- Jean-Claude Lacaille, PhD, Université de Montréal, Montréal, Canada
- Gabor Szabo, PhD, Institute of Experimental Medicine, Budapest, Hungary
- Lisa Topolnik, PhD, Université de Montréal, Montréal, Canada
Contact
For more information, email mcbainc@mail.nih.gov or visit http://neuroscience.nih.gov/Lab.asp?Org_ID=124.



