You are here: Home > Unit on Developmental Neuroethology
Neuroethology of Crying

- John D. Newman, PhD, Head, Unit on Developmental Neuroethology *
- Deborah Bernhards, BS, Biological Technician
Crying during infancy is a familiar behavior, known to parents and other caregivers for the compelling nature of the signal, and important to physicians and the medical research community for the evidence suggesting a link between crying (particularly, the acoustic details of the cry sounds) and behavioral and neurological measures of normal and subnormal trajectories of development. This project has provided evidence that the detailed acoustic structure of infant crying reflects a universal mammalian behavior and has aimed to determine the neural circuitry underlying infant crying and cry responding by caregivers in non-human primates.
Our overall goals of this project are to (1) understand the changes in vocal behavior that accompany development, paying particular attention to the roles of individual experience and heritable traits that may influence these developmental changes; (2) develop new methodology for analyzing the acoustic details of infant cries and other vocalizations; (3) understand the mechanisms underlying infant crying in a non-human primate—the common marmoset. We are particularly interested in defining the neural circuitry responsible for cry production and the neural circuitry activated in parents and other caregivers when hearing and responding to a crying infant. Our studies employ methods of immunocytochemistry, neuroanatomy, and functional gene expression in the brain to define the specific populations of neurons underlying these behaviors, the genes that are activated in the course of building the relevant functional brain circuits, and the roles of developmental age and experience in these processes. We are also interested in determining the critical acoustic features of cry sounds that activate caregiving behavior. To this end, we employ computer-based analysis of cry sounds to track the details of crying at different ages, as well as behavioral methods aimed at addressing the differential responsiveness of listerners of various hormonal states and experience that may influence responsiveness.
Genetic approaches to understanding the evolution of auditory communication
Prosimian primates are the most ancient primate line, and have been separated evolutionarily from other primates for millions of years. Analysis of the cry sounds of prosimians may give clues to the early evolutionary history of auditory communication in primates. To this end, the vocalizations of infant prosimians representing the main prosimian groups (lemurs and lorises) were analyzed. We found that members of the lorises (galagos, bushbabies) produced click-like sounds when separated from their mother or nest. These sounds are markedly different from the long, tonal sounds of simian primates and suggests that early in the evolutionary history of primates, the predominantly nocturnal species produced sounds that promoted localization in the absence of visual cues. Lemurs, on the other hand, produce longer infant cries, more like simian cries. However, only the ringtailed lemur, Lemur catta, produces tonal cries. The members of the Lemur fulvus clade produce noisy cries, which are easier to localize. This suggests that early primate evolution was marked by experimentation with various acoustic subtypes of infant vocalization.
In a collaboration with the Evolutionary Biology Program at Cornell University, we have begun to explore the relationship between vocal behavior and phylogeny, using bioacoustic and genetic analyses of individual species. The initial effort focuses on a group of songbirds, the warblers (family Parulidae). DNA analysis of the phylogenetic relationships of warblers conducted at Cornell provides the basis for examining the extent to which vocal characteristics are an accurate reflection of phylogenetic affinities. The Macaulay Library at the Cornell Laboratory of Ornithology, which possesses the largest collection of digital recordings of animal sounds in the world, provides the source of material for this analysis. Digital recordings of nearly 100 species of warblers, all of which have had DNA samples analyzed, have been downloaded, and analysis, using software developed at Cornell, has begun.
Click image to enlarge.
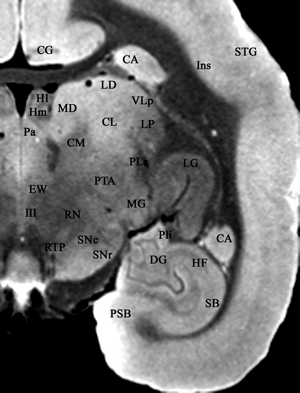
Figure 1. MRI image from female marmoset, with labeled brain structures
Key: CA = caudate nucleus; CG = cingulate gyrus; CL = central lateral nucleus; CM = center median nucleus; DG = dentate gyrus; EW = Edinger-Westphal nucleus; HF = hippocampal formation; HI = lateral habenular nucleus; HM = medial habenular nucleus; III = oculomotor nucleus; Ins = insula; LD = lateral dorsal nucleus; LG = lateral geniculate nucleus; MD = mediodorsal nucleus; Pa = paraventricular nucleus; Pla = anterior nucleus of the pulvinar; Pli = inferior nucleus of the pulvinar; PSB = presubiculum; RN = red nucleus; RTP = reticulotegmental pontine nucleus; SB = subiculum; SNc = pars compacta of substantia nigra; SNr = pars reticulate of substantia nigra; STG = superior temporal gyrus; VLp = ventral lateral posterior nucleus. MRI image provided by Nicholas Bock.
Neuroanatomy of infant crying and cry responding
To identify the brain structures mediating infant crying and cry responding by adults, we are using c-fos immunocytochemistry following the appropriate behavioral context. To facilitate identifying structures containing Fos, the protein product of the c-fos gene, we have produced a combined histological and MRI atlas of the marmoset brain. A representative MRI section from the atlas is shown in Figure 1.
Publications
- Newman JD, Harris JC. The scientific contributions of Paul D. MacLean. J Nerv Ment Dis 2009 197:3-5
- Newman JD. Neurobiology of the parental brain. J Nerv Ment Dis 2009 197:555
- Newman JD, Kenkel WM, Aronoff EC,Bock NA, Zametkin MR, Silva AC. A combined histological and MRI brain atlas of the common marmoset monkey, Callithrix jacchus. Brain Res Rev 2009 [Epub ahead of print]
Collaborators
- Nicholas A. Bock, PhD, Laboratory of Functional and Molecular Imaging, NINDS, Bethesda, MD
- Irby Lovette, PhD, Cornell University, Ithaca, NY
- Afonso C. Silva, PhD, Laboratory of Functional and Molecular Imaging, NINDS, Bethesda, MD
- James T. Winslow, PhD, Neurobiology Non-Human Primate Core, NIMH, Bethesda, MD
Contact
For more information, email jn1g@nih.gov



