You are here: Home > Section on Membrane Biology
Membrane Fusion Mediated by Protein Fusogens

- Leonid V. Chernomordik, PhD, Head, Section on Membrane Biology
- Eugenia Leikina, DVM, Senior Research Assistant
- Kamram Melikov, PhD, Staff Scientist
- Jean-Philippe Richard, PhD, Visiting Fellow
- Samristha Sanyal, PhD, Visiting Fellow
- Sung-Tae Yang, PhD, Visiting Fellow
- Elena Zaitseva, PhD, Research Fellow
- Sergei A. Pourmal, Summer Student
Disparate membrane remodeling reactions are tightly controlled by protein machinery but are also dependent on the lipid composition of the membranes. Whereas each kind of protein has its own individual personality, membrane lipid bilayers have rather general properties manifested by their resistance to disruption and bending. Our long-term goal is to understand how proteins remodel membrane lipid bilayers in important cell biology processes. The starting point for our analysis is a consideration of the physical factors that determine the tendency of the membrane bilayers to change their topology. We expect that the analysis of the molecular mechanisms of important and diverse membrane rearrangements will bring about new ways of controlling them and clarify the generality of emerging mechanistic insights. In our most recent studies, we focused on mechanisms of the fusion stage of dengue virus (DEN) infection and escape of cationic cell-penetrating peptides (CPP) from endosomes; we found that a negatively charged lipid bis(monoacylglycero)phosphate (BMP), which is highly enriched in late endosomes, drastically facilitates both DEN fusion and CPP translocation. Our findings emphasize the importance of the unusual lipid composition of late endosomes in the intracellular localization of diverse membrane remodeling events.
Dengue virus ensures its fusion in late endosomes using compartment-specific lipids.
DEN, the most prevalent mosquito-borne virus world-wide, is endemic in more than 100 countries, with an estimated 100 million cases of DEN infections per year. Annually, 90% of the 500,000 hospitalizations are pediatric. Due to climate change and the increased mobility of people across national borders, this infectious tropical disease is an ever-growing global threat to health and the economy. Dengue hemorrhagic fever is a leading cause of death among children in some Asian countries. Currently, there are neither vaccines nor effective therapies for DEN infections. Similar to many other viruses, cell-bound and then internalized DEN delivers its genome into cells by fusion between viral membrane and endosomal membrane. The fusion stage of viral entry is an important target for antivirals (including the HIV-1 fusion inhibitor enfuvirtide). However, screening of potential antivirals of this class requires application of fusion assays such as virus-mediated cell fusion or virus fusion to protein-free model membranes (liposomes). Surprisingly, development of such assays for DEN has been unsuccessful. Also surprisingly, DEN has been reported to fuse only in late endosomes, while activation of the DEN protein fusogen glycoprotein E is triggered already at a pH characteristic for early endosomes.
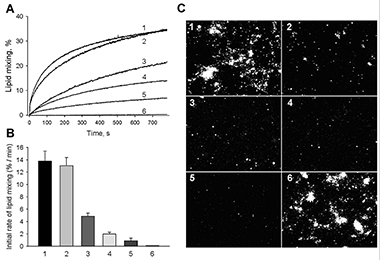
Figure 1. The dependence of dengue virus fusion on anionic lipids determines intracellular localization of viral RNA release.
Cell surface–bound and then internalized dengue virus travels through acidified early endosomes of mammalian cells and fuses only in late endosomes, where the virus encounters anionic lipids for the first time during entry. As a result, virus effectively delivers its RNA to intracellular replication sites. (click image to enlarge)
We were interested in determining whether there are any cofactors required for DEN fusion that would dictate that fusion does not occur until the virion enters late endosomes and whether such cofactors had been missing in earlier attempts to achieve DEN fusion to plasma membrane of mammalian cells and liposomes. We found that DEN uses BMP, a lipid specific to late endosomes, as a co-factor for its endosomal acidification-dependent fusion machinery. Effective virus fusion to plasma and intracellular membranes, as well as to protein-free liposomes, requires that the target membrane contains anionic lipids such as BMP and phosphatidylserine. Anionic lipids act downstream of low pH–dependent fusion stages and promote the advance from the earliest hemifusion intermediates to the fusion pore opening. To reach anionic lipid-enriched late endosomes, DEN travels through acidified early endosomes (Figure 1), but we found that the low pH-dependent loss of fusogenic properties of DEN is relatively slow in the presence of target membranes that are free of anionic lipids. We propose that anionic-lipid dependence of the DEN fusion machinery protects it against premature irreversible restructuring and inactivation and ensures viral fusion in late endosomes, where the virus encounters anionic lipids for the first time during entry. As a result, DEN effectively delivers viral RNA to its translation/replication sites. Identification of the essential cofactor of the DEN fusion machinery allowed us to develop an arsenal of fusion assays (virus-liposome fusion, virus-cell fusion and DEN intracellular fusion). The assays developed in this study to directly characterize DEN fusion will hopefully help in developing antivirals such as ones targeting DEN interactions with anionic lipids to block or prematurely activate viral fusion machinery. Furthermore, interactions between the protein fusogen of DEN protein E and anionic lipids may have implications for the pathogenesis of dengue hemorrhagic fever, which is characterized by activation of endothelial cells and extracellular exposure of anionic lipids.
A cell-penetrating peptide induces leaky fusion of liposomes containing late endosome–specific anionic lipid.
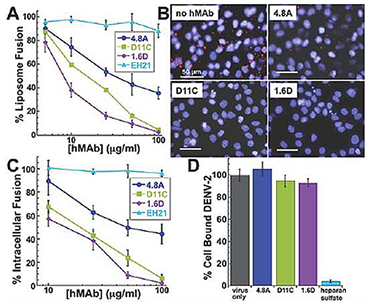
Figure 2. The proposed mechanism of intracellular entry for cationic cell-penetrating peptides
Cell-penetrating peptides (CPPs, red) are internalized through diverse pathways of endocytosis. The low content of anionic phospholipids in the leaflets of the plasma membrane and early endosomes that are exposed to CPPs minimizes peptide interactions with the lipid bilayers of these membranes. However, once CPPs reach the lumen of a multivesicular late endosome, they bind to intraluminal vesicles that are enriched in bis(monoacylglycero)phosphate (BMP, shown in blue), an anionic lipid specific to late endosomes. CPP-mediated leaky fusion between these vesicles allows the peptide to enter the lumen of intraluminal vesicles. Finally, the back fusion of intraluminal vesicles with limiting membrane of late endosome releases CPP into cytosol. (click image to enlarge)
Using model lipid bilayers, we explored mechanisms of delivery of cationic cell-penetrating peptides into cytosol. CPPs such as the TAT peptide and other arginine-rich peptides are a promising vehicle for the delivery of macromolecular drugs. While many studies indicate that CPPs enter cells by endocytosis, the mechanisms by which they cross endosomal membranes remain elusive. Moreover, some papers indicate that endosomal escape is a limiting factor in endocytosis-dependent delivery of functionally active CPP-cargo conjugates into the cytosol and nucleus. Thus, understanding the mechanism of the endosomal escape is of great practical importance.
In our recent work, we proposed a new model for the delivery of cationic CPPs and conjugated cargo into cytosol (Figure 2). The model is based on both the characteristic multivesicular morphology of late endosomes and the fact that intraluminal vesicles are highly enriched with the late endosome-specific anionic lipid BMP. We hypothesize that cationic peptides induce leaky fusion (i.e., fusion associated with membrane permeabilization) between intraluminal vesicles, resulting in delivery of the peptide and cargo molecules into intraluminal vesicles. Subsequent back fusion of intraluminal vesicle with the limiting membrane of late endosomes releases peptide and cargo into the cell cytosol. To test this model we focused on interactions of TAT peptide with model protein-free lipid bilayers—liposomes of various lipid compositions including those mimicking lipid bilayers of the plasma membrane and late endosomes. We report that the TAT peptide induces leakage of encapsulated probes and translocates across BMP-enriched bilayers that mimic the lipid composition of intraluminal vesicles of late endosome but not across membranes that mimic the lipid composition of plasma membranes. TAT-induced leakage and translocation are linked to membrane fusion and inhibited by fusion inhibitors. These results substantiate the proposed model of endosomal escape and suggest that membrane fusion, known to be an important stage of intracellular delivery for membrane-enclosed macromolecules such as viral nucleic acids, may also be involved in the delivery of membrane-free macromolecules.
Additional Funding
- NIAID's 2009-2011 Trans-NIH Biodefense Program
Publications
- Rafikova ER, Melikov K, Ramos C, Dye L, Chernomordik LV. Transmembrane protein-free membranes fuse into Xenopus nuclear envelope and promote assembly of functional pores. J Biol Chem. 2009; 284:29847-29859.
- Kozlov MM, McMahon HT, Chernomordik LV. Protein-driven membrane stresses in fusion and fission. Trends Biochem Sci. 2010; in press.
- Zaitseva EM, Yang S, Melikov K, Pourmal SA, Chernomordik LV. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog. 2010; 6:e1001131. doi:10.1371.
- Yang S, Zaitseva EM, Chernomordik LV, Melikov K. Cell penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid. Biophys J. 2010; in press.
- Rafikova ER, Melikov K, Chernomordik LV. Cytosol dependent membrane fusion in ER, nuclear envelope and nuclear pore assembly: biological implications. Nucleus. 2010; in press.
Collaborators
- Ori Avi-Noam, MS, Technion-Israel Institute of Technology, Haifa, Israel
- Louis Dye, BS, Microscopy and Imaging Core Facility, NICHD, Bethesda, MD
- Michael M. Kozlov, PhD, DHabil, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- Benjamin Podbilewicz, PhD, Technion-Israel Institute of Technology, Haifa, Israel
- Corinne Ramos, PhD, Division of Biological Sciences, University of California, San Diego, La Jolla, CA
- Elvira Rafikova, PhD, University of California Santa Cruz, Santa Cruz, CA
Contact
For more information, email chernoml@mail.nih.gov.


