You are here: Home > Section on Environmental Gene Regulation
Small Regulatory RNAs and Small Proteins

- Gisela Storz, PhD, Head, Section on Environmental Gene Regulation
- Aixia Zhang, PhD, Staff Scientist
- Shantanu Bhatt, PhD, Postdoctoral Fellow
- Michael D. Dambach, PhD, Postdoctoral Fellow
- Yue Hao, PhD, Postdoctoral Fellow
- Taylor B. Updegrove, PhD, Postdoctoral Fellow
- Lauren S. Waters, PhD, Postdoctoral Fellow
- Melissa Sandoval, BS, Postbaccalaureate Student
- Maureen K. Thomason, MS, Graduate Student
- Xuefeng Yin, BS, Graduate Student
Currently, we have two main interests: the identification and characterization of small noncoding RNAs and the identification and characterization of small proteins of less than 50 amino acids. Both small RNAs and small proteins have been overlooked because they are not detected in biochemical assays and the corresponding genes are poorly annotated and missed in genetic screens. However, mounting evidence suggests that both classes of these small molecules play important regulatory roles.
Identification and characterization of small regulatory RNAs
During the past 15 years, we carried out several different systematic screens for small regulatory RNA genes in E. coli. These screens, which included computational screens for conservation of intergenic regions and direct detection after size selection or co-immunoprecipitation with the RNA-binding protein Hfq, are all applicable to other organisms. We are now examining small RNA expression using tiled microarrays together with deep sequencing to further extend our identification of small RNAs, particularly antisense RNAs.
A major focus of the group has been to elucidate the functions of the small RNAs we and others identified. Early on, we showed that the OxyS RNA, whose expression is induced in response to oxidative stress, acts to repress translation through limited base pairing with target mRNAs. We discovered that the OxyS action is dependent on the Sm-like Hfq protein, which functions as a chaperone to facilitate OxyS RNA base pairing with its target mRNAs. We also found that another abundant and broadly conserved small RNA mimics the DNA structure of an open promoter and modulates RNA polymerase activity.
It is now clear that Hfq-binding small RNAs, which act through limited base pairing, are integral to many different stress responses in E. coli. We showed that MicC, whose expression is induced in minimal medium and at low temperature, represses translation of the OmpC outer membrane porin. We also reported that FnrS, whose expression is induced by FNR upon a shift from aerobic to anaerobic conditions, acts to downregulate the levels of a variety of mRNAs encoding metabolic enzymes. In both examples, the small RNA represses the synthesis of proteins and enzymes that are not needed under conditions when levels of the small RNA are highest.
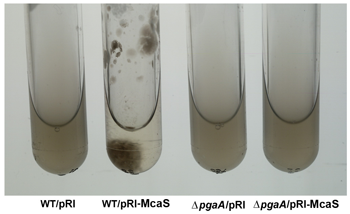
Click image to enlarge.
Figure 1. The McaS small RNA induces auto-aggregation of cells.
While cells carrying the vector control (first and third tubes from left) remain suspended, cells overexpressing the McaS small RNA (second tube from left) stick to the walls and sediment to the bottom of the test tube in the absence of shaking. The phenotype is attributable to McaS activation of the pgaABCD operon required for the synthesis and export of poly-b-1,6-N-acetyl-D-glucosamine (PGA) and thus is not is not observed in the pgaA deletion strain (fourth tube from left).
In more recent work, we discovered that the McaS RNA, whose levels are elevated in stationary phase or when glucose is limiting, regulates mRNA targets involved in various aspects of biofilm formation. McaS represses csgD, the transcription regulator of curli biogenesis, and activates flhD, the master transcription regulator of flagella synthesis, leading to increased motility, a process not previously reported to be regulated by sRNAs. McaS also regulates pgaA, a porin required for the export of the polysaccharide poly β-1,6-N-acetyl-D-glucosamine (Figure 1). Consequently, high levels of McaS result in increased biofilm formation while a strain lacking mcaS shows reduced biofilm formation. Based on these observations, we propose that McaS modulates steps in the progression to a sessile lifestyle.
Other recent studies showed that the Spot 42 RNA, whose levels are highest when glucose is present, plays a broad role in catabolite repression by directly repressing genes involved in central and secondary metabolism, redox balancing, and the consumption of diverse nonpreferred carbon sources. Many of the genes repressed by Spot 42 are transcriptionally activated by the global regulator CRP. Because CRP represses Spot 42, the regulators participate in a specific regulatory circuit called a multi-output feedforward loop. We found that the loop can reduce leaky expression of target genes in the presence of glucose and can maintain repression of target genes under changing nutrient conditions. Our results suggest that base-pairing RNAs in feedforward loops can help shape the steady-state levels and dynamics of gene expression.
In addition to small RNAs that act via limited base pairing, we have become interested in small antisense RNAs that have the potential to form extensive base-pairing interactions with their mRNA targets encoded on the opposite strand. We previously showed that base pairing between the GadY RNA and the 3′ untranslated region of the gadX mRNA encoded opposite GadY leads to increased levels of the gadX mRNA and GadX protein. Recently, we demonstrated that gadX is transcribed in an operon with gadW and that base pairing of GadY with the gadXW mRNA results in processing, giving rise to two halves that accumulate to higher levels than the full length mRNA. Multiple enzymes, including the double strand RNA-specific endoribonuclease RNase III, are involved in the GadY-direct cleavage.
We also reported that a large class of antisense RNAs acts to repress the synthesis of small toxic proteins. For example, in characterizing the Sib RNAs, which are encoded by five repeats in E. coli K-12, we observed an overexpression phenotype reminiscent of plasmid addiction. Further examination of the SIB repeat sequences revealed conserved open reading frames encoding highly hydrophobic 18-19 amino acid proteins (Ibs) opposite each sib gene. The Ibs proteins were found to be toxic when overexpressed, and the toxicity could be prevented by co-expression of the corresponding Sib RNA. Two other RNAs encoded divergently in another intergenic region were similarly found to encode a small hydrophobic protein (ShoB) and an antisense RNA regulator (OhsC). Computational screens together with experimental validation showed that these small hydrophobic protein-antisense RNA gene modules, termed type 1 toxin-antitoxin modules, are much more widely distributed among bacteria than previously appreciated.
Studies are under way to characterize further other Hfq-binding RNAs and antisense RNAs and to elucidate the roles of small RNAs that act in ways other than base pairing.
Identification and characterization of small proteins
In our genome-wide screens for small RNAs, we found that several short RNAs do encode small proteins. The correct annotation of the smallest proteins is one of the biggest challenges of genome annotation, and perhaps more importantly, few annotated short ORFs have been confirmed to correspond to synthesized proteins. Although these proteins have largely been missed, the few small proteins that have been studied in detail in bacterial and mammalian cells were shown to have important functions in signaling and in cellular defenses. We thus established a project to identify and characterize E. coli proteins of less than 50 amino acids.
We used sequence conservation and ribosome binding site models to predict genes encoding small proteins, defined as having 16-50 amino acids, in the intergenic regions of the E. coli genome. We tested expression of the predicted as well as previously annotated small proteins by integrating the sequential peptide affinity tag directly upstream of the stop codon on the chromosome and assaying for synthesis using immunoblot assays. The approach confirmed that 20 previously annotated and 18 newly discovered proteins of 16-50 amino acids are synthesized.
Remarkably, more than half the newly discovered proteins are predicted to be single transmembrane proteins. This observation prompted us to examine the localization, topology, and membrane insertion of the small proteins. Biochemical fractionation showed that, consistent with the predicted transmembrane helix, the small proteins are generally most abundant in the inner membrane fraction. Examples of both Nin-Cout and Nout-Cin orientations were found in assays of topology reporter fusions to representative small transmembrane proteins. Interestingly however, three of nine tested proteins display dual topology. Positive residues close to the transmembrane domains are conserved, and mutational analysis of one small protein, YohP, showed that the positive inside rule applies for single transmembrane domain proteins, as has been observed for larger proteins. Finally, fractionation analysis of small protein localization in strains depleted of the Sec or YidC membrane insertion pathways revealed differential requirements. Some small proteins appear to be affected by both Sec and YidC depletion, others showed more dependence on one or the other insertion pathway, while one protein was not affected by depletion of either Sec or YidC. Thus, despite their diminutive size, small proteins display considerable diversity in topology, biochemical features, and insertion pathways.
To elucidate the functions of the small proteins, we now are employing many of the approaches the group has used to characterize the functions of small regulatory RNAs. Systematic assays for the accumulation of tagged versions of the proteins have shown that many small proteins accumulate under specific growth conditions or after exposure to stress. We also generated and screened bar-coded null mutants and identified small proteins required for resistance to cell envelope stress and acid shock. In addition, the attached sequential peptide affinity tag is being exploited to identify co-purifying complexes. The combination of these approaches is giving insights into when, where, and how the small proteins are acting.
We recently showed that expression of a 42-amino acid protein, now denoted MntS (formerly the small RNA gene rybA), is repressed by manganese through MntR. Overproduction of MntS causes manganese sensitivity while a lack of MntS perturbs proper manganese-dependent repression of another manganese-regulated gene. Based on these results, we propose that MntS plays a novel role in intra-cellular manganese trafficking and homeostasis.
We also found that the 49-amino acid inner membrane protein AcrZ (formerly named YbhT) associates with the AcrAB-TolC multidrug efflux pump, which confers resistance to a wide variety of antibiotics and other compounds in E. coli. Co-purification of AcrZ with AcrB (in the absence of both AcrA and TolC), two-hybrid assays, and suppressor mutations indicate that this interaction occurs through the inner membrane protein AcrB. The highly-conserved acrZ gene is co-regulated with acrAB through induction by the MarA, Rob, and SoxS transcription regulators. In addition, mutants lacking AcrZ are sensitive to many of, but not all, the antibiotics transported by AcrAB-TolC. The differential antibiotic sensitivity suggests that AcrZ may enhance the ability of the AcrAB-TolC pump to export certain classes of substrates.
Publications
- Hobbs EC, Yin X, Paul BJ, Astarita JL, Storz G. Conserved small protein associates with the AcrB efflux pump and differentially affects antibiotic resistance. Proc Natl Acad Sci USA 2012;109:16696-16701.
- Thomason MK, Fointaine F, De Lay N, Storz G. A small RNA that regulates motility and biofilm formation in response to changes in nutrient availability in Escherichia coli. Mol Microbiol 2012;84:17-35.
- Beisel CL, Updegrove TB, Janson BJ, Storz G. Multiple factors dictate target selection by Hfq-binding small RNAs. EMBO J 2012;31:1961-1974.
Collaborators
- Susan Gottesman, PhD, Laboratory of Molecular Biology, NCI, Bethesda, MD
Contact
For more information, email storz@helix.nih.gov or visit www.nichd.nih.gov/research/atNICHD/Investigators/storz.

