You are here: Home > Section on Organelle Biology
Interplay between Membrane Organelles, Cytoskeleton and Metabolism in Cell Organization and Function

- Jennifer Lippincott-Schwartz, PhD, Head, Section on Organelle Biology
- Dylan Burnette, PhD, Postdoctoral Fellow
- Antony Chen, PhD, Postdoctoral Fellow
- Uri Manor, PhD, Postdoctoral Fellow
- Carolyn Ott, PhD, Postdoctoral Fellow
- Angelika Rambold, PhD, Postdoctoral Fellow
- Prasanna Satpute, PhD, Postdoctoral Fellow
- Prabuddha Sengupta, PhD, Postdoctoral Fellow
- Arnold Seo, PhD, Postdoctoral Fellow
- Alex Valm, PhD, Postdoctoral Fellow
- Schuyler Van Engelenburg, PhD, Postdoctoral Fellow
- Pick Wei, PhD, Postdoctoral Fellow
- Yu Chen, PhD, Visiting Fellow
- Sarah Cohen, PhD, Visiting Fellow
- Alex Ritter, BA, Graduate Student
We investigate the global principles underlying cell behavior at both small and large spatial scales. At the small scale, we employ the super-resolution imaging techniques of photoactivated localization microscopy (PALM), interferometric 3D PALM, single particle tracking PALM, and pair correlation PALM to map out the spatial organization, stoichiometry, and dynamics of proteins associated with various membrane-bound compartments and the cytoskeleton. We also employ fluorescence photobleaching, photoactivation, fluorescence correlation, and fluorescence energy transfer methods to measure protein-protein interactions, protein turnover rates, and protein association rates. These approaches allow us to assay cellular functions, including receptor stoichiometry and protein clustering and diffusion behavior at the nanometric scale in living cells. At the large scale, we are investigating how complex behaviors of cells arise, such as cell crawling (Figure 1), polarization, furrowing, cytokinesis, cell fate determination, viral budding, and intercellular transfer. We study these intricate behaviors by quantitatively analyzing diverse intracellular processes, including membrane trafficking, autophagy, actin/microtubule dynamics, and organelle assembly/disassembly pathways, which undergo dramatic changes as cells alter their behavior and organization throughout life. To assist with these efforts, we combine various fluorescence-based imaging approaches, including TIRF imaging and spinning-disk and laser-scanning confocal microscopy, with FRAP, FLIP, and photoactivation to obtain large image data sets. We process the data sets computationally to extract biochemical and biophysical parameters, which can be related to results from conventional biochemical assays. We then use the results to generate mechanistic understanding and predictive models of the behavior of cells and subcellular structures (including ER, Golgi, cilia, endosomes, lysosomes, autophagosomes, and mitochondria [Figure 2]) under healthy and pathological conditions.
DRP1–dependent mitochondrial fission initiates follicle cell differentiation during fly oogenesis.
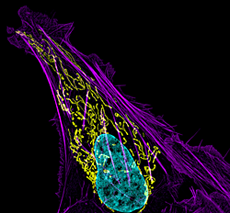
Click image to enlarge.
Figure 1. Mitochondral and actin organization in a crawling cell
SIM image of MITO-RFP, Phalloidin-Alexa 488, and DNA (Hoeschst 33342) in a U2OS cell (osteoblastoma)
We demonstrated a regulatory link between mitochondrial fission activity and cell cycle exit as the follicle cell layer develops during Drosophila melanogaster oogenesis. We accomplished this by performing live-cell imaging after manipulating key mitochondrial fission/fusion proteins. We observed that posterior-localized clonal cells in the follicle cell layer of developing ovarioles with down-regulated expression of the major mitochondrial fission protein DRP1 did not exit the cell cycle. Instead, they excessively proliferated, failed to activate Notch for differentiation, and exhibited downstream developmental defects. Reintroduction of mitochondrial fission activity or inhibition of the mitochondrial fusion protein Marf-1 in DRP1–null clones reversed the block in Notch-dependent differentiation. When DRP1–driven mitochondrial fission activity was unopposed by fusion activity in Marf-1–depleted clones, premature cell differentiation of follicle cells occurred in mitotic stages. Our findings thus revealed that DRP1–dependent mitochondrial fission activity regulates the onset of follicle cell differentiation during Drosophila oogenesis. The results link, for the first time, mitochondrial dynamics and cell fate determination.
Multiscale diffusion in the mitotic fly syncytial blastoderm
The effects of the geometry of the early syncytial Drosophila embryo on the effective diffusivity of cytoplasmic proteins are poorly understood. Using a combination of mathematical/computational modeling and live-cell imaging, we characterized the effects of dynamic syncytial geometry on the effective diffusivity of cytoplasmic proteins in the Drosophila syncytial blastoderm. We found that the presence of transient mitotic membrane furrows results in a multiscale diffusion effect that has a significant impact on effective diffusion rates across the embryo. Based on these results, we proposed that one role of syncytial membrane furrows is to temporally regulate bulk embryonic diffusion rates to balance the multiscale effect of interphase nuclei, which ultimately stabilizes the shapes of various morphogen gradients.
Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes.
Rab proteins are important regulators of insulin-stimulated GLUT4 translocation to the plasma membrane (PM), but the precise steps in GLUT4 trafficking modulated by particular Rab proteins remain unclear. To clarify this issue, we used TIRF microscopy to monitor GLUT4 trafficking under basal conditions and during insulin signaling, identifying Rab proteins associated with GLUT4 storage vesicles (GSVs) and their mode of action on GLUT4 trafficking. Screening a large set of Rab proteins for colocalization with GLUT4, for association with IRAP–pHluorin vesicles fusing at the PM in response to insulin stimulation, and for PM recruitment under insulin stimulation, we discovered that only Rab10 associates with GSVs and mediates GSV peripheral translocation after becoming activated. The Rab GTPase activation protein AS160 negatively regulated Rab10 activity on GSVs. Furthermore, we found that Rab10 interacted with the actin motor protein myosin-Va. This facilitated the translocation of GSVs to sites at the PM where the vesicles could fuse. High expression levels of a short tail form of myosin-Va lacking the actin-binding domain reduced GLUT4 delivery to the PM. Thus, even though multiple Rab proteins regulate the trafficking of GLUT4, it is only Rab10 in coordination with myosin-Va that mediates the final steps of GSV translocation to the PM under insulin stimulation.
Computational model of cytokinetic abscission driven by ESCRT-III polymerization and remodeling

Click image to enlarge.
Figure 2. Mitochondria morphology in NRK cells
Image, obtained by structured illumination microscopy, of mitochondria labeled with GFP-tagged prohibitin
The physical separation of two daughter cells at the end of mitosis, known as cytokinetic abscission, involves cleavage of a narrow, microtubule-based, intercellular bridge that connects two nascent daughter cells arising during cell division. We have been investigating the role of the endosomal sorting complex required for transport (ESCRT)-III complex in this process. Using high-resolution, quantitative imaging of ESCRT-III during cytokinetic abscission, we observed that ESCRT-III initially assembles at the midbody dark zone and then polymerizes outward to the site of cytokinetic abscission. Integrating these observations with the known biophysical properties of ESCRT-III complexes, we then formulated and tested a computational model for ESCRT–mediated cytokinetic abscission. In this model, ESCRT-III forms a fission complex that drives constriction and abscission of the intercellular cytokinetic bridge. The ESCRT-III fission complex arises as a result of breakage enabled by the ATPase VPS4 of the initial ESCRT-III oligomer, polymerizing at the edge of the midbody dark zone. Once formed, the fission complex constricts to its spontaneous diameter of about 50 nm, while sliding along the intercellular bridge away from the midbody dark zone. Sliding continues until the fission complex reaches its equilibrium position corresponding to the minimal elastic energy of the bridge membrane. The subsequent abscission is driven by attachment of the bridge membrane to the dome-like end-cap of the fission complex. We substantiated this model by theoretical analysis of the membrane elastic energy and by experimental verification of the major model assumptions. Currently, we are exploring whether ESCRT-III polymerization coupled with breakage and sliding of a constricting membrane-bound ESCRT-III complex to a location corresponding to minimal elastic energy of the membrane is a conserved property of other ESCRT–mediated processes (i.e., human immunodeficiency virus budding).
Bleaching/blinking-assisted localization microscopy for super-resolution imaging using standard fluorescent molecules
Super-resolution imaging techniques based on the precise localization of single molecules, such as photoactivated localization microscopy (PALM), achieves high resolution by fitting images of single fluorescent molecules that have been photoactivated with a theoretical Gaussian to localize them with a precision on the order of tens of nanometers. To simplify the technique, we developed a new way of producing point localization–based super-resolution images that does not require photoactivatable or photoswitching probes. Called bleaching/blinking-assisted localization microscopy (BaLM), the technique relies on the intrinsic bleaching and blinking behaviors characteristic of all commonly used fluorescent probes. The approach detects single fluorophores by acquiring a stream of fluorescence images. Fluorophore bleach or blink-off events are then detected by subtracting from each image of the series the subsequent image. Similarly, blink-on events are detected by subtracting from each frame the previous one. After image subtractions, fluorescence emission signals from single fluorophores are identified and the localizations determined by fitting the fluorescence intensity distribution with a theoretical Gaussian. BaLM works with all commonly used synthetic fluorescent dyes and genetically expressed fluorescent proteins. Thus, BaLM can be readily used in multicolor super-resolution experiments, mapping out the molecular distribution of up to four different proteins in a sample. Furthermore, BaLM allows for molecules to be localized by similar algorithms already used for the analysis of PALM data. As such, BaLM offers a practical and versatile approach for obtaining super-resolution images that can stand alone or be used in conjunction with other super-resolution approaches.
Primary cilia utilize glycoprotein-dependent adhesion mechanisms to stabilize long-lasting cilia-cilia contacts.
Primary cilia have major roles in sensing and transmitting information into cells. We hypothesized that like motile cilia, primary cilia have the potential to form adhesions. To test this, we examined cilia in two different tissues: photoreceptors in the retina and cholangiocytes in the liver. In both these environments we observed that cilia form contacts with each other. Using a cell-culture model combined with fluorescent cell imaging, we demonstrated that cilia from nearby cells could form persistent, regulated, glycoprotein-dependent cilia-cilia adhesions. In addition, we found evidence for cellular control of adhesion release. We suggest that, like the contacts made by motile cilia, adhesion of primary cilia is functionally relevant. The results also suggest that mammalian primary cilia may be more than passive, solitary receivers.
Plasticity of the asialoglycoprotein receptor deciphered by ensemble FRET imaging and single-molecule counting PALM imaging
The stoichiometry and composition of membrane protein receptors are critical to their function. However, the inability to assess receptor subunit stoichiometry in situ has hampered efforts to relate receptor structures to functional states. In this study, we deciphered the oligomeric state of the asialoglycoprotein receptor using ensemble FRET imaging, analytical modeling, and single-molecule counting with photoactivated localization microscopy (PALM). We found that the two subunits of the asialoglycoprotein receptor (rat hepatic lectin 1 [RHL1] and RHL2) can assemble into both homo- and hetero-oligomeric complexes, displaying three forms with distinct ligand specificities that coexist on the plasma membrane. These include higher-order homo-oligomers of RHL1, higher-order hetero-oligomers of RHL1 and RHL2 with two-to-one stoichiometry, and homo-dimer RHL2 with little tendency to further homo-oligomerize. We demonstrated that levels of the complexes can be modulated in the plasma membrane by exogenous ligands. Our results demonstrate that even a simple two-subunit receptor can exhibit remarkable plasticity in structure and consequently, function.
Additional Funding
- IATAP (Intramural AIDS Targeted Antiviral Program)
Publications
- Chen Y, Wang Y, Zhang J, Deng Y, Jiang L, Song E, Wu XS, Hammer JA, Xu T, Lippincott-Schwartz J. Rab10 and myosin-Va coordinately mediate insulin-stimulated GLUT4 storage vesicle translocation to the plasma membrane in adipocytes. J Cell Biol 2012;198:545-560.
- Elia N, Fabrikant G, Kozlov MM, Lippincott-Schwartz J. Computational model of cytokinetic abscission driven by ESCRT-III polymerization and remodeling. Biophysics J 2012;102:2309-2320.
- Burnette DT, Sengupta P, Dai Y, Lippincott-Schwartz J, Kachar B. Bleaching/blinking assisted localization microscopy for superresolution imaging using standard fluorescent molecules. Proc Natl Acad USA 2011;108:21081-21086.
- Mitra K, Rikhy R, Lilly M, Lippincott-Schwartz J. DRP1-dependent mitochondrial fission initiates follicle cell differentiation during during Drosophila oogenesis. J Cell Biol 2012;197:487-497.
- Daniels BR, Rikhy R, Renz M, Dobrowsky T, Lippincott-Schwartz J. Multiscale diffusion in the mitotic Drosophila melanogastor syncytial blastoderm. Proc Natl Acad USA 2012;109:8588-8593.
Collaborators
- Michael Davidson, PhD, Florida State University, Tallahassee, FL
- Bechara Kachar, PhD, Laboratory of Cell Structure and Dynamics, NIDCD, Bethesda, MD
- Michael Kozlov, PhD, Tel Aviv University, Tel Aviv, Israel
Contact
For more information, email lippincj@mail.nih.gov or visit lippincottschwartzlab.nichd.nih.gov.


