You are here: Home > Section on Gamete Development
Cell Cycle Regulation in Oogenesis

- Mary Lilly, PhD, Head, Section on Gamete Development
- John Reich, PhD, Postdoctoral Fellow
- Bradley Reveal, PhD, Postdoctoral Fellow
- Tanveer Akbar, PhD, Visiting Fellow
- Weili Cai, PhD, Visiting Fellow
- Youheng Wei, PhD, Visiting Fellow
- Kuikwon Kim, MS, Technician
The long-term goal of our laboratory is to understand how the cell-cycle events of meiosis are coordinated with the developmental events of gametogenesis. Chromosome mis-segregation during female meiosis is the leading cause of miscarriages and birth defects in humans. Recent evidence suggests that many meiotic errors occur downstream of defects in oocyte growth and/or the hormonal signaling pathways that drive differentiation of the oocyte. Thus, an understanding of how meiotic progression and gamete differentiation are coordinated during oogenesis is essential to studies in both reproductive biology and medicine. We use the genetically tractable model organism Drosophila melanogaster to examine how meiotic progression is instructed by the developmental and metabolic program of the egg.
In mammals, studies on the early stages of oogenesis face serious technical challenges in that entry into the meiotic cycle, meiotic recombination, and the initiation of the highly conserved prophase I arrest all occur during embryogenesis. In contrast, in Drosophila these critical events of early oogenesis all take place continuously within the adult female. Easy access to the early stages of oogenesis, coupled with available genetic and molecular genetic tools, makes Drosophila an excellent model for studies on meiotic progression and oocyte development.
To understand the regulatory inputs that control early meiotic progression, we are working to determine how the oocyte initiates and then maintains the meiotic cycle within the challenging environment of the ovarian cyst. Our studies focus on questions that are relevant to the development of all animal oocytes. What strategies does the oocyte use to protect itself from inappropriate DNA replication? How does the oocyte inhibit mitotic activity before meiotic maturation and the full growth and development of the egg? How does cell-cycle and metabolic status within the ovarian cyst influence the differentiation of the oocyte? To answer these questions, we have undertaken studies to determine the basic cell-cycle program of the developing ovarian cyst.
The SEA/GATOR complex: integrating developmental and metabolic signals in oogenesis
Click image to enlarge.
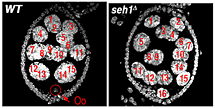
Figure 1. The nucleoporin Seh1 is required for maintenance of the meiotic cycle.
Wild-type (WT) and seh1Δ egg chambers stained with the DNA dye DAPI. The seh1Δ egg chamber has 16 polyploid nurse cells but no oocyte (Oo).
TORC1 is a primary regulator of cell growth and metabolism that responds to multiple signals including nutrient availability, energy status, and growth factors. We determined that components of the SEA/GATOR complex define a new upstream module of TORC1 regulation in Drosophila that responds to amino-acid scarcity and plays an essential role in the regulation of oocyte growth and meiotic maintenance. From our studies, we conclude that the tight regulation of TORC1 activity by the SEA/GATOR complex is critical to oocyte development and physiology. Moreover, our data strongly suggest that the important role of nutrient stress pathways in the regulation of gametogenesis has been conserved from single-cell to multicellular animals.
The meiotic regulators Mio and Seh1 are components of the SEA/GATOR complex
In earlier studies, we identified two genes, missing oocyte (mio) and seh1, that regulate meiotic progression and the maintenance of the oocyte fate. Both mio and seh1 are highly conserved from yeast to humans. In mio and seh1 null mutants, oocytes enter the meiotic cycle and progress to pachytene. The meiotic cycle, however, is not maintained. Ultimately, a large fraction of mio and seh1 oocytes withdraw from meiosis, enter the endocycle, and become polyploid (Figure 1). Genetic and phenotypic analyses indicate that mio and seh1 act early in oogenesis, before the formation of the synaptonemal complex (SC) and meiotic recombination. Using biochemical strategies, we determined that Mio and Seh1 are components of a large multiprotein complex called the Seh1-associated (SEA) complex in yeast and the Gap Activity Towards Rags (GATOR) complex in higher eukaryotes. Recently, the SEA/GATOR complex was shown to be an upstream regulator of TORC1 activity. We found that TORC1 kinase activity is dramatically reduced in the ovaries from mio and seh1 null mutants. Reduced TORC1 activity correlates with the strong reduction in growth observed in mio- and seh1-mutant ovaries. In contrast, mio and seh1 appear to have little effect on TORC1 activity and growth in most somatic tissues. Thus, there is a unique tissue-specific requirement for the TORC1 activators Mio and Seh1 in the female germline.
Npr2 and Npr3 mediate an essential response to amino acid starvation in Drosophila:
Click image to enlarge.
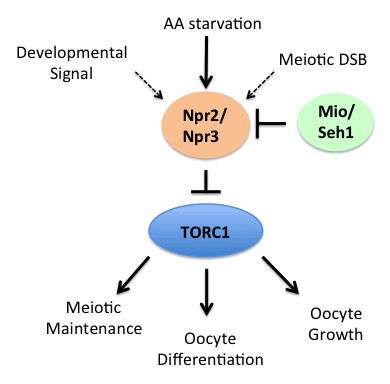
Figure 2. The SEA/GATOR complex, an upstream regulator of TORC1, controls oocyte development and meiotic progression in Drosophila.
The SEACAT/GATOR2 components Mio and Seh1 function to inhibit the activity of the SEACIT/GATOR1 components Npr2 and Npr3. In mio and seh1 mutants, the constitutive activity of Npr2 and Npr3 results in the unchecked inhibition of TORC1 activity and a disruption in oocyte growth and development.
We determined that the SEA/GATOR complex members Mio and Seh1 physically and genetically interact with the TORC1 inhibitors Npr2 and Npr3. Intriguingly, in yeast Npr2 and Npr3 inhibit TORC1 in response to amino acid starvation and are required for sporulation and meiotic progression. However, the precise role of Npr2 and Npr3 in the development and physiology of multicellular organisms remained mostly unexplored. Over the last year, we determined that Npr2 and Npr3 mediate an adaptive response to amino acid limitation in developing oocytes. The response is essential to the preservation of female fertility during times of protein scarcity. When npr2 or npr3 were knocked down in the female germline, egg chambers underwent apoptosis in response to amino acid limitation. The TORC1 inhibitor rapamycin rescued this apoptotic phenotype. Thus, an important protective function of Npr2/Npr3 in the female germline lies in their ability to downregulate TORC1 activity. Our results demonstrate that, in metazoans, which have evolved alternative pathways to regulate TORC1, the basic physiological requirement for Npr2/Npr3 in the response to amino acid starvation has been retained.
The TSC1/2 complex is a TORC1 inhibitor that is required to maintain baseline levels of TORC1 activity in most cell types. As observed with npr2 and npr3, we found that depleting Tsc1 in the female germline renders oogenesis acutely sensitive to amino acid stress. Yet the TSC1/2 complex is not activated by amino acid starvation. One possible model to explain these data is that, in the female germline, the TSC1/2 complex is required to maintain baseline levels of TORC1 activity while Npr2/Npr3 are required to specifically downregulate TORC1 activity in response to amino acid scarcity. Therefore, both TORC1 inhibitor pathways are required to repress TORC1 activity in order to prevent oocytes from being shunted into the apoptotic pathway in response to amino acid scarcity. We predict that, in many tissues, the two independent TORC1–inhibitory pathways work in concert to fine-tune TORC1 activity in response to various developmental and environmental inputs.
Mio and Seh1 oppose the activity of TORC1 inhibitors Npr2/Npr3 in the female germline:
Using genetic epistasis analysis, we determined that, during the development of the Drosophila oocyte, the TORC1 activators Mio and Seh1 are required to oppose the activity of the TORC1 inhibitors Npr2 and Npr3 (Figure 2). In mio and seh1 mutants, TORC1 activity is constitutively repressed in the germline of developing egg chambers, resulting in the activation of catabolic metabolism and a block to meiotic progression and oocyte development. Notably, the mio and seh1 ovarian phenotypes can be rescued either through depleting npr2 or npr3 in the female germline or by raising baseline levels of TORC1 activity by disabling the TSC1/2 complex. The data confirm that the failure to maintain the meiotic cycle and the oocyte fate in mio and seh1 mutants is a direct result of inappropriately low TORC1 activity in the female germline. Thus, as is observed in single-celled eukaryotes, the tight regulation of TORC1 activity by the SEA/GATOR complex guides cells through the early meiotic program and gametogenesis in Drosophila.
Publications
- Kassis JA, Lilly MA. PRC2 goes solo in the Drosophila female germline. Dev Cell 2013;26:329-330.
- Mitra K, Rikhy R, Lilly M, Lippincott-Schwartz J. DRP1-dependent mitochondrial fission initiates follicle cell differentiation during Drosophila oogenesis. J Cell Biol 2012;197:487-497.
Collaborators
- Chi-Hon Lee, MD, PhD, Program in Cellular Regulation and Metabolism, NICHD, Bethesda, MD
- Jennifer Lippincott-Schwartz, PhD, Cell Biology and Metabolism Program, NICHD, Bethesda, MD
- Kim McKim, PhD, Waksman Institute of Microbiology, Rutgers, The State University of NJ, Piscataway, NJ
- Juan Riesgo-Escovar, PhD, Universidad Nacional Autonoma de Mexico, Queretaro, Mexico
Contact
For more information, email mlilly@helix.nih.gov or visit cbmp.nichd.nih.gov/uccr.

