You are here: Home > Section on Intercellular Interactions
Pathogenesis of HIV-1 and its Copathogens in Human Tissues

- Leonid Margolis, PhD, Head, Section on Intercellular Interactions
- Jean-Charles Grivel, PhD, Staff Scientist
- Christophe Vanpouille, PhD, Staff Scientist
- Anush Arakelyan, PhD, Visiting Fellow
- Victor Silva, PhD, Visiting Fellow
- Sonia Zicary, PhD, Visiting Fellow
- Wendy Fitzgerald, BS, Technician
The general goal of the Section of Intercellular Interactions is to understand the mechanisms of pathogenesis of human pathogens, including the human immunodeficiency virus (HIV). Given that the critical events of this infection occur in tissues, we used for our study a system of human tissues ex vivo, developed in our Section and now adopted by many investigators, to study viral infections and to test antivirals. During the last year in the framework of the general goal of our Section, we focused on three aims: (i) to translate in vivo our earlier ex vivo observation on HIV-1 suppression by the common anti-herpetic drug acyclovir; (ii) to develop and to test in ex vivo human tissues new anti-HIV-1 antivirals that are based on the newly synthesized heterodimer of the widely used anti-HIV-1 drugs AZT and 3TC; and (iii) to extend our flow virometry technology to study the antigenic composition of individual extracellular vesicles that are now widely recognized as mediators in cell-cell communications in norm and pathology.
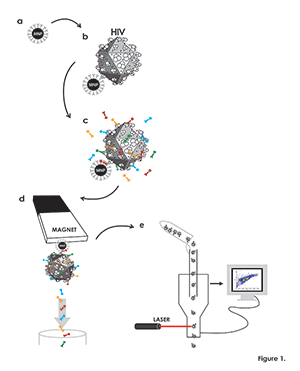
Figure 1. Flow virometry procedure
(a) Magnetic nanoparticles (MNPs) coupled to a virion-specific antibody against gp120 (“capture antibody”), (grey). (b) MNPs coupled to capture antibody are incubated with viruses (schematically presented as icosahedrons), which become immobilized on MNPs. (c) MNP–immobilized viruses are visualized with human anti-gp120 antibody (turquoise) recognizing a different epitope from that recognized by the capture antibody. MNPs are visualized with a fluorescent antibody or its Fab (red) against the Fc portion of the capture antibody, and viral antigens of interest are visualized with fluorescently labeled monoclonal antibodies (green and yellow). (d) Virus–MNP complexes with bound antibodies are separated from unbound antibodies on magnetic columns. (e) The MNP–immobilized virions stained with fluorescence-labeled antibodies and eluted from the magnetic columns are analyzed with the flow cytometer set up to trigger on MNP fluorescence rather than on light scatter.
The research conducted during the last year provided new insights into HIV-1 transmission and pathogenesis, leading to new concepts in anti-HIV-1 strategies.
The anti-herpetic drug acyclovir as an anti-HIV drug
Acyclovir (ACV) is a highly specific antiherpetic drug, currently widely used particularly against herpes simplex virus 2 (HSV-2) infection. HSV-2 is among the most common co-pathogens in HIV-1–infected persons, and it establishes with HIV-1 a vicious circle in which each virus facilitates the replication, shedding, and acquisition of the other. ACV is commonly used in HIV-1/HSV-2 coinfected patients to treat symptomatic HSV-2 infection. Several retrospective studies found that ACV treatment of HIV-1–infected individuals was associated with increased survival. These early observations were then corroborated by numerous more recent randomized trials, which demonstrated that HSV-2–suppressive therapy using ACV (or its prodrug valacyclovir, valACV) reduces plasma HIV-1 RNA concentration by 0.25 to 1.23 logs in HIV-1/HSV-2–coinfected persons and delays HIV-1 disease progression. In view of the fact that ACV was always believed to be inactive against HIV-1, the results were explained by a reduction in generalized inflammation owing to the suppression of HSV-2. Surprisingly, we found that, in ex vivo human tissues, ACV suppresses HIV-1 by directly inhibiting HIV-1 reverse transcriptase, provided that ACV is phosphorylated by the thymidine kinase of Herpesviruses (HHVs) present in these tissues. We hypothesized that, if a similar mechanism occurs in vivo, the suppressive effect of ACV on HIV-1 replication should not be limited to HSV-2–coinfected individuals. We tested this hypothesis in a randomized, placebo-controlled, cross-over trial. The trial aimed to evaluate the impact of valACV on HIV-1 viremia in HSV-2–seronegative individuals not on antiretroviral therapy. Twenty-one HIV-infected male and female subjects were divided in two groups. The groups were well balanced with regard to demographic characteristics, CD4+ T cell count, and plasma HIV RNA level at baseline. There were no significant imbalances by study site other than ethnic background. Group A received 12 weeks of valACV 500 mg given twice daily by mouth, followed by two weeks of no treatment, then 12 weeks of placebo; Group B received 12 weeks of placebo followed by 12 weeks of valACV. We found that valACV reduced HIV-1 levels in plasma by 0.40 log10 copies/ml, demonstrating that ACV can suppress HIV-1 replication in the absence of HSV-2 coinfection. Plasma HIV-1 concentrations rebounded to pre-enrollment levels within two weeks of termination of valACV treatment, consistent with the short half-life of the drug and its lack of intracellular accumulation. Although the effect of valACV on HIV-1 RNA is modest, it is comparable to monotherapy with other antiretrovirals (e.g., zidovudine or stavudine monotherapy), and the reduction of HIV-1 plasma viral load mediated by ACV could be clinically beneficial. Given that mathematical modeling suggests that progression to an AIDS–defining illness or death declines by 25% for every 0.3 log10 decrement in plasma HIV RNA. The reduction of HIV-1 load observed in our study provides evidence that the effect of ACV on HIV-1 load is not restricted to the presence of HSV-2 and suggests that ACV has a direct anti HIV-1 activity in vivo. In light of the ACV clinical data, an ACV chronic suppressive regimen may be beneficial for HIV-1–infected patients whether or not co-infected with HSV-2.
Development of new anti-HIV compounds
Despite intensive research to find new drugs, nucleoside reverse transcriptase inhibitors (NRTIs) remain at the central core of HIV-1 treatment. Among eight NRTIs that have been used, the most extensively studied is 3-azido-3-deoxythymidine (AZT, zidovudine, retrovir). Although AZT has been widely used since the beginning of antiretroviral era, the drug has significant side effects. In particular, zidovudine induces mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. Also, upon AZT monotherapy, resistant virions are quickly selected. In particular, five mutations in HIV reverse transcriptase (RT) contributing to the development of high-level resistance to zidovudine have been described. Phosphonate derivatives of AZT showed significantly lower toxicity and improvement of AZT therapeutic properties. Similar to AZT phosphonate derivatives, 2′,3′-dideoxy-3′-thiacytidine (3TC, lamivudine, Epivir) 5-H-phosphonate and 3TC 5-aminocarbonylphosphonate were found to be much less toxic than parent 3TC in cell cultures. Also, in laboratory animals, prodrug transformation into the active nucleoside 3TC was slower, thus making phosphonate derivatives of NRTIs promising candidates as extended-release forms of the parent NRTI. We described a new phosphonate derivative, namely 3TC-AZT heterodimer, a chimera of AZT-5- and 3TC-5-aminocarbonylphosphonate. At this early stage of development of the new antiviral, we characterized its structure and tested potential antiviral activity of the newly synthesized phosphonate 3TC-AZT chimera.
Given that, in vivo, the critical pathogenic events of HIV infection occur in tissues that are not faithfully reflected by single cell cultures, we utilized a system of human lymphoid tissue ex vivo that retains tissue cytoarchitecture and was developed in our laboratory. The system supports HIV-1 replication and has been shown to complement pre-clinical drug testing against various pathogens. Furthermore, such a system reflects the in vivo donor-to-donor variability and allows testing of various drugs as a preliminary step before engaging in costly clinical trials. Blocks of human tonsillar tissue were treated with phosphonate derivatives overnight and then infected with a prototypical HIV-1 X4LAI.04. The compounds were present during the entire culture period. We found that the 3TC-AZT heterodimer significantly suppressed HIV-1 replication at the level that surpasses some of the clinically used antivirals. Moreover, it exhibited low toxicity towards various tissue lymphocytes.
We thus showed that the development of bisphosphonate derivatives is feasible and that a bisphosphonate of 3TC and AZT inhibited HIV-1 in human lymphoid tissue. Its low toxicity and its complex metabolism, which is associated with a slow release of active compounds, make it a candidate for future development and demonstrate that the phosphonate strategy in general, and the bisphosphonate derivatives in particular, may be useful for the development of heterodimers of anti-HIV-1 compounds.
Antigenic composition of individual extracellular vesicles (EVs)
Microvesicles, exosomes, and apoptotic bodies play an important role in cell-to-cell communication because different proteins, lipids and RNAs are specifically incorporated into these vesicles, which can be targeted to remote cells through receptor-ligand interactions. Release of EVs is part of normal physiological processes and is reported to change in pathologies. Given that various cells supplying EVs to blood express different antigens, EVs produced by these cells are antigenically distinct. Analyses of blood EV composition, which have been performed predominantly in bulk, have revealed the presence of various cellular antigens in EVs but could not reflect the distribution of these antigens on individual EVs, although such distribution may reflect the physiological conditions of the donor. Furthermore, conventional flow cytometry cannot be applied to analysis of small particles such as EVs.
We developed a new nanoparticle-based technique for analysis of surface proteins on single blood nano-sized (less than 300 nm) EVs. We used a regular commercial flow cytometer and magnetic nano particles (MNPs) to isolate fluorescence-labeled EVs and to separate them from non-bound fluorescent antibodies. Moreover, the analysis can be performed not only on EVs released by cells in culture, but also directly on blood plasma EVs. With MNPs coupled to antibodies against EV antigens, it is possible to focus on minor fractions of EVs, which contain few vesicles per microliter, out of the large numbers EVs that are reported for normal blood plasma. We were also able to evaluate the distribution of antigens in these minor EV fractions. In particular, when we captured CD31–carrying EVs, we found that about half the EVs co-expressed CD41 and CD63. Although CD63 is a highly prevalent antigen, our fine-analysis of antigen distribution demonstrated that the antigen is not carried by approximately 20% of the CD31+CD41+ EVs; thus these EVs form a separate fraction.
In conclusion, we performed a fine-analysis of single blood EVs according to the distribution of antigens they carry. We demonstrated that the blood EV population is a mosaic, with various EVs carrying different combinations of antigens. None of the antigens can be claimed to be present on all EVs. The results would be impossible to obtain in a bulk analysis, which reports only on the general presence or absence of particular antigens in EV preparations.
Because of the reproducibility of our analysis of distributions of individual blood EVs according to the combinations of antigens they carry, it is now possible to relate the distributions to the medical condition of an individual donor, as well as to search for EV–antigenic patterns common to particular diseases. We are now using the technique to analyze antigenic composition of EVs associated with pathologic pregnancies and cardio-vascular diseases as well as with HIV infection.
Additional Funding
- Intramural-to-Russia (I-to-R) Program
Publications
- Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, Grivel J, Singh S, Margolis LB. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis 2012;205:97-105.
- Lisco A, Introini A, Munawwar A, Vanpouile C, Grivel J-C, Blank P, Singh S, Margolis LB. HIV-1 imposes rigidity on blood and semen cytokine network. Am J Reprod Immunol 2012;68:515-521.
- Introini A, Vanpouille C, Lisco A, Grivel J-C, Margolis L. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathogens 2013;9:1-10.
- Saba E, Origoni M, Taccagni G, Ferrari, D, Doglioni C, Nava A, Lisco A, Grivel J-C, Margolis L, Poli G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol 2013;Epub ahead of print.
- Arakelyan A, Fitzgerald W, Margolis L, Grivel J-C. Flow virometry: a nanoparticle based technology for analysis of individual viral particles. J Clin Invest 2013;123:3716-3727.
Collaborators
- Jan Balzarini, PhD, Rega Institute, Katholieke Universiteit Leuven, Leuven, Belgium
- Sarman Singh, MD, All India Institute of Medical Sciences, New Delhi, India
Contact
For more information, email margolis@helix.nih.gov.