You are here: Home > Molecular Neurophysiology and Biophysics Unit
Potassium Channels and Dendritic Function in Hippocampal Pyramidal Neurons

- Dax Hoffman, PhD, Head, Molecular Neurophysiology and Biophysics Unit
- Emilie Campanac, PhD, Visiting Fellow
- Begum Choudhury, MS, Biologist
- Eunyoung Kim, PhD, Postdoctoral Fellow
- Lin Lin, PhD, Research Fellow
- Michael W. Nestor, PhD, Postdoctoral Fellow
- Wei Sun, BS, Graduate Student
- Michael Hatch, BS, Postbaccalaureate Fellow
- Ben Throesch, BS, Postbaccalaureate Fellow
The central nervous system underlies all our experiences, actions, emotions, knowledge, and memories. With billions of neurons each firing hundreds of times per second, the complexity of the brain is stunning. To pare down the task of understanding something so complex, our research approach calls for studying the workings of a single central neuron—the pyramidal neuron from the CA1 region of the hippocampus. The hippocampus is essential for long-term memory in humans and is among the first brain regions affected by epilepsy and Alzheimer's disease. To understand how the hippocampus stores and processes information, we focus on one of its principal cell types, the CA1 pyramidal neuron. Each pyramidal neuron in the CA1 region of the hippocampus receives tens of thousands of inputs onto its dendrites, and it is commonly thought that information is stored by altering the strength of individual synapses (synaptic plasticity). Recent evidence suggests that the regulation of synaptic surface expression of glutamate receptors can, in part, determine synaptic strength. However, the dendrites contain an abundance of ion channels that are involved in receiving, transforming, and relaying information in the dendrites, adding an additional layer of complexity to neuronal information processing.
We have found that the A-type potassium channel subunit Kv4.2 is highly expressed in the dendritic regions of CA1 neurons in the hippocampus and, as one of the primary regulators of dendritic excitability, plays a pivotal role in information processing. Kv4.2 is targeted for modulation during the types of plasticity thought to underlie learning and memory. Moreover, the functional expression level of Kv4.2 was found to regulate the subtype expression of the NMDA–type glutamate receptors, the predominate molecular devices controlling synaptic plasticity and memory. We are currently following up on these findings with more detailed investigations into the mechanisms of activity-dependent Kv4.2 regulation. In addition, we have begun investigating the role of dendritic voltage-gated channels in CNS disorders, including autism-spectrum disorder and Alzheimer's disease, that exhibit profound changes in neuronal excitability along with learning and memory deficits.
Kv4.2 trafficking in CA1 pyramidal neuron dendrites
We previously reported that neuronal stimulation results in a redistribution of Kv4.2 channels away from dendritic spines to the dendritic shaft. This activity-dependent redistribution of Kv4.2 required activation of NMDA–type glutamate receptors and calcium influx, two requirements shared with synaptic plasticity, which is thought to underlie learning and memory. Given the nonuniform distribution of Kv4.2 channels in CA1 dendrites, Mike Nestor performed experiments to test the hypothesis that Kv4.2 channels are differentially trafficked at different regions along the apical dendrite during basal activity and upon stimulation in CA1 neurons. Proximal (50–150 µm from the soma, primary and oblique) and distal (>200 µm) apical dendrites were selected. The fluorescence recovery after photobleaching (FRAP) technique was used to measure basal cycling rates of EGFP-tagged Kv4.2 (Kv4.2g). We found that the cycling rate of Kv4.2 channels was one order of magnitude slower at both primary and oblique dendrites between 50–150 µm from the soma. Kv4.2 channel cycling increased significantly at 200–250 µm from the soma. Expression of a Kv4.2 mutant lacking a phosphorylation site for protein kinase-A (Kv4.2gS552A) abolished this distance-dependent change in channel cycling, demonstrating that phosphorylation by PKA underlies the increased mobility in distal dendrites. Neuronal stimulation increased cycling of Kv4.2 channels significantly at distal sites only. The activity-dependent increase in Kv4.2 cycling at distal dendrites was blocked by expression of Kv4.2gS552A. The results indicate that distance-dependent Kv4.2 mobility is regulated by activity-dependent phosphorylation of Kv4.2 by PKA.
Functional role of the Kv4.2 auxiliary subunit AKAP
A potential source of PKA modulation of Kv4.2 was uncovered this year by research fellow Lin Lin and graduate student Wei Sun when they identified the A-kinase–anchoring protein (AKAP) as a novel accessory subunit for Kv4.2. AKAPs target PKA to glutamate receptor and ion channel complexes to allow for discrete, local signaling. We determined that the C-terminal domain of Kv4.2 interacts with an internal region of AKAP79/150 that overlaps with its MAGUK binding domain. AKAP79/150–anchored PKA activity was shown to control Kv4.2 surface expression in heterologous cells and hippocampal neurons. Consistent with these findings, we found that disrupting PKA leads to a decrease in neuronal excitability while preventing dephosphorylation by the phosphatase calcineurin results in increased excitability. The results demonstrate that AKAP79/150 provides a platform for dynamic PKA regulation of Kv4.2 expression, fundamentally impacting neuronal excitability.
Functional role of the Kv4.2 auxiliary subunit KChIP4a
KChIP (KChIP1–4) associates with the N-terminal of Kv4.2 and modulates the channel's biophysical properties, turnover rate, and surface expression. We investigated the role of the Kv4.2 C-terminal PKA phosphorylation site S552 in the KChIP4a-mediated effects on Kv4.2 channel trafficking. We found that, while interaction between Kv4.2 and KChIP4a does not require PKA phosphorylation of Kv4.2S552, phosphorylation of this site is necessary for both enhanced stabilization and membrane expression of Kv4.2 channel complexes produced by KChIP4a. Enhanced surface expression and protein stability conferred by co-expression of Kv4.2 with other KChIP isoforms did not require PKA phosphorylation of Kv4.2 S552. These data demonstrate that PKA phosphorylation of Kv4.2 plays an important role through its specific interaction with KChIP4a.
Functional role of the Kv4.2 auxiliary subunit DPP6
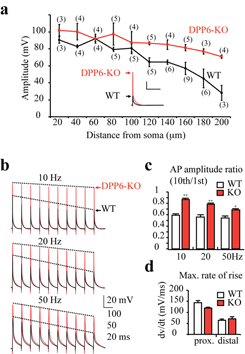
Click image to enlarge.
Figure 1. Enhanced dendritic excitability in DPPP6-KO mice
(a) Back-propagating action potentials (bAPs) recorded in distal CA1 DPP6-KO dendrites (red) were larger in amplitude than those recorded in dendrites from WT mice (black). In control recordings, bAP amplitude decreased with distance from the soma. Although bAP amplitude also slightly decreased in DPP6-KO recordings, significant differences in bAP amplitude from WT began at distances more than 100 μm from soma. The (#) indicates the number of patches. Scale bars: 20mV, 200 ms. Inset. Example bAP traces recorded from 180μm distal dendrite. (b) Example traces of action potential trains elicited with 10, 20, and 50Hz antidromic stimulation. All recordings were made ~ 160 μm from soma. (c) Pooled data show that bAP amplitude exhibits a relatively small activity-dependent decrease in DPP6-KO recordings compared with the much larger reduction in WT controls (p<0.05). (d) Although bAPs propagated better in DPP6-KO vs. WT recordings, no significant changes in the maximal rate of rise (an estimate of Na+ channel activity) were found.
Studies in heterologous expression systems have shown that Kv4 alpha-subunits interact with transmembrane proteins to regulate channel trafficking and properties. The DPP6 auxiliary subunit protein, which is expressed in CA1 neurons, has recently been identified in large copy-number variants' screens from some populations as an Autism Spectrum Disorder and ALS target gene. DPP6 enhances the opening probability of Kv4 channels and increases channel surface expression in heterologous systems. Using dendritic recordings from DPP6 knockout mice, graduate student Wei Sun discovered that DPP6 is critical for generating the A-type K+ current gradient observed in CA1 dendrites. The loss this gradient led to hyper-excitable dendrites, with implications for information storage and coding (see Figure 1). Additional, preliminary results show a critical role for DPP6 in synapse formation during development. We are currently investigating the possibility, suggested by these results, that dendritic excitability might be a commonly affected factor in CNS disorders recently associated with the DPP6 gene.
Role of Kv4.2 channels in synaptic plasticity and development
We found that altering functional Kv4.2 expression level leads to a rapid, bidirectional remodeling of CA1 synapses. Neurons exhibiting enhanced A-type K+ current (IA) showed a decrease in relative synaptic NR2B/NR2A subunit composition and did not exhibit a form of synaptic plasticity called long-term potentiation (LTP). Conversely, reducing IA by expression of a Kv4.2 dominant negative or genomic knockout of Kv4.2 led to an increased fraction of synaptic NR2B/NR2A and enhanced LTP. Our data suggest that A-type K+ channels are an integral part of a synaptic complex that regulates Ca2+ signaling through spontaneous NMDA receptor activation to control synaptic NMDA receptor expression and plasticity. Additional advances included an investigation into the role of Kv4.2 in controlling the expression of synaptic NMDA receptors in vivo and during development. Synaptic NR2B fraction is developmentally regulated with implications for synaptic plasticity and learning and memory as well as diseases associated with learning impairments. Eunyoung Kim found that in vivo injection of a virus to alter Kv4.2 expression levels bidirectionally regulates NR2B subunit expression throughout development.
Dendritic intrinsic plasticity in memory and disease
We previously demonstrated a role of A-type K+ channels in regulating intrinsic excitability of CA1 pyramidal neurons of the hippocampus after the induction of synaptic plasticity. This non-synaptic plasticity, called intrinsic plasticity, might represent an information storage mechanism available to neurons in addition to synaptic plasticity. Emilie Campanac is attempting to dissociate the signals involved in the induction of synaptic and intrinsic plasticity by the use of GluA1–lacking mice. In CA1 pyramidal neurons, the GluA1 subunit is differentially recruited by different patterns of activity known to induce long-term potentiation.
Intrinsic plasticity has also been observed upon drug addiction. The psychostimulant effects of the addictive drug cocaine are attributed to inhibition of the dopamine transporter, which increases dopaminergic transmission. Chronic exposure to cocaine leads to neuro-adaptations in several voltage membrane conductances of neurons localized in the medial prefrontal cortex (mPCF) and nucleus accumbens. To date, these modifications have all been characterized in the soma. Our goal is to identify more precisely which conductances are regulated in dendrites. Adult male mice were injected for five consecutive days with cocaine or saline. No significant difference was observed in intrinsic excitability in pyramidal neurons of the mPCF after cocaine injection. We did, however, find a left shift in the EPSP–spike coupling curve after cocaine injection. The shift is abolished in the presence of an inhibitor of GABAA receptors, suggesting a decrease of inhibition after cocaine.
Publications
- Lin L, Sun W, Wikenheiser AM, Kung F, Hoffman DA. KChIP4a regulates Kv4.2 channel trafficking through PKA phosphorylation. Mol Cell Neurosci. 2010;43(3):315-25.
- Shaw, M, Hammond, RS and Hoffman DA. Dendritic Ion Channel Trafficking and Plasticity. Trends in Neuroscience 2010;33(7):307-16.
- Nestor MW, Hoffman DA. Differential cycling rates of Kv4.2 channels in proximal and distal dendrites of hippocampal CA1 pyramidal neurons. Hippocampus 2010;In Press.
- Lin L, Sun W, Kung F, Dell'Acqua ML, Hoffman DA. AKAP79/150 impacts intrinsic excitability of hippocampal neurons through phospho-regulation of A-type K+ channel trafficking. J Neurosci. 2011;26;31(4):1323-32.
- Sun W, Lin L, Maffie, JK, Petralia, RS, Rudy B and Hoffman DA. DPP6 establishes the A-type K+ current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 2011;In Press.
Collaborator
- Johannes Hell, PhD, University of California Davis, Davis, CA
- Andrew Holmes, PhD, Laboratory for Integrative Neuroscience, NIAAA, Rockville, MD
- Bernardo Rudy, MD, PhD, New York University Medical Center, New York, NY
- Constantine A. Stratakis, MD, D(Med)Sc, Program in Devlopmental Endocrinology and Genetics, NICHD, Bethesda, MD
Contact
For more information, email buonanno@mail.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/andres-buonanno.aspx.

